Table of Contents

This book is dedicated to the many mycologists who helped to uncover the secrets behind the life cycle of these little mushrooms, and to the Mazatec peoples of Mexico, who have for centuries protected, nourished, and handed down the ceremony, knowledge, and wisdom they reveal.
We would also like to thank Kat for having so generously agreed to produce a new Psilocybe cubensis life cycle illustration. Her beautiful artwork illuminated the pages of the book that germinated our mycological careers, and it is a great honor to have some of that same light grace our own little book.
Finally, we would like to thank Mellea R. Millaria, our little honey mushroom, for her keen photographic eye, patience, and beauty. Without her unwavering support, this book would surely never have fruited.
PROLOGUE
In 1992, while perusing the dusty aisles of a Manhattan antiquarian book-shop, we happened upon a dog-eared copy of O.T. Oss and O.N. Oeric’s Psilocybin: Magic Mushroom Grower’s Guide. This slim volume, with its densely packed text and fanciful, otherworldly line drawings, held for us an immediate and irresistible allure. Like an illuminated manuscript or a book of spells, it glimmered and hummed with meaning, reaching out to us from the crowded shelves. It seemed less a book than a communiqué, a missive cast out into the world, waiting silently for years to at last make its way into our hands. We already held a considerable affection for the mushrooms in question, but we had never before contemplated growing our own.Yet by the time we exited the shop, book in hand, the idea seemed self-evident, organic: Of course, we thought, we will grow our own mushrooms!
For us, this book has never lost its intense personal appeal, but we were hardly the sole intended recipients of the secrets it contained. First published in 1976, Psilocybin has been in print ever since and has sold over 150,000 copies. The methods it espouses have inspired the careers of untold numbers of mushroom cultivators and kitchen mycologists (your humble authors among them), and sparked a flurry of underground experimentation and innovation. Although several books and pamphlets on the subject of psilocybin mushroom cultivation have been published before and since Psilocybin: Magic Mushroom Grower’s Guide, this book is unique in a number of important ways.
First of all, it presents a series of methods that can be performed by nearly anyone, requiring only a limited investment in specialized tools and materials, such as a pressure cooker and Petri dishes. Second, unlike previously available techniques, Oss and Oeric’s methodology is relatively simple, reliable, and quite productive. Though they did not invent any of the methods they espoused, they were the first to combine them into such an efficient and effective system. Finally, it is far more than a simple manual for the cultivation of psilocybin mushrooms. With its philosophical asides, lovely, phantasmagorical illustrations, and Lovecraftian speculations about the off-world origins of the organisms and their import for humankind (including a statement of purpose supposedly dictated to one of the authors by the mushroom overlords themselves!), it is, above all, a great read.
Although Psilocybin: Magic Mushroom Grower’s Guide is a classic, a new manual on psilocybin mushroom cultivation is nonetheless needed. While Psilocybin has stood the test of time as literature, it has become obsolete as a grower’s guide. As easy and reliable as the Oss & Oeric method was, it still left a great deal of room for improvement. In the 30 years since Psilocybin first appeared, many cultivation techniques have been considerably refined or supplanted entirely, and a number of new technological and mycological discoveries have been made.
The purpose of this book is to update and complement the methods Oss and Oeric described. It is our hope that by creating a new resource for beginning Psilocybe mushroom cultivators, the burden of being such a resource will be lifted from that book. Psilocbyin Mushroom Handbook updates the outmoded technical information within its predecessor’s pages, leaving Psilocybin: Magic Mushroom Grower’s Guide free to be seen for what it is: a work of art. It is our humble wish that our efforts here will provide concise and well organized instructions for mushroom cultivation that incorporate the most up-to-date practices. In so doing, we hope to help keep in print the book that first sparked our mushroom imagination to life so it will continue to inspire new students of psycho-mycology for years to come.
INTRODUCTION
If you spend a little time perusing the published literature or the various Web sites on the subject of Psilocybe mushroom cultivation, you will quickly notice the dizzying array of methods that one can use to grow these mushrooms.This is particularly true for Psilocybe cubensis: there are the “Psilocybe Fanaticus Technique,” methods utilizing wild bird seed, composted cow or horse manure, worm castings, and so on. As you will come to see, this is a very robust species with which to work, easily adaptable to a wide variety of substrates and conditions. As a result, it is the ultimate “tinkerer’s” mushroom, and has inspired countless experiments in search of the best, newest, or simply the wackiest1 method to make it fruit.
Such “primary research” stands as one of the paramount joys of working in science, but it can also be its greatest frustration, particularly for a beginner. Some 99.99% of scientific investigations result in setbacks, dead ends, or outright failure, and that is exactly how it should be. Only by process of elimination does one arrive at that elusive, precious 0.01% Holy Grail of success (proving, in the end, that failures aren’t really failures anyway). Anyone who has spent any amount of time doing scientific research eventually becomes comfortable with this seemingly skewed ratio. One comes to see failures as simply part of the process, even sometimes a welcome part, since there is usually much to be learned from something that doesn’t work.
Nevertheless, for a beginner this can be a difficult lesson to learn. Early failures (often among the most catastrophic) can be so disheartening for the novice that she is inclined to give up completely. More than a few times we were ourselves ready to chuck it all and go back to doing something easy, like brain surgery. But then we discovered that brain surgery wasn’t nearly as much fun, returned to growing mushrooms, and eventually our successes were more spectacular than our failures. We can assure you that the same will hold true for you if you stick with it despite whatever setbacks you may encounter along the way.
We have tried to present to the reader a set of mushroom cultivation methods that are simple and reliable enough to at least minimize the number of problems and failures that might arise. It is a system that is, if not foolproof, at least fool-resistant. We have sought to avoid methods that are confusing or present too many choices for the cultivator at each stage of the process. Instead, we have tried to guide the novice from one end of the mushroom life cycle to the other in the simplest and most direct route possible.
The methods we present are among those that we have found the simplest and most effective in our hands.You should not interpret the omission of any other methods from this guide as an implicit critique of their merits. Time and space prevent us from describing or commenting on all the possible ways you might grow these mushrooms.You could very likely find success using one of these alternative methods, and we would never want to dissuade you from further experimentation, if that is your desire.
This book is not meant to be the final word about psilocybin-containing mushrooms. Its main purpose is simply to expose the beginner to basic and reliable methods for growing several of them. The thing about beginners is that once they get going, they don’t remain beginners for long, and soon outgrow their initial training. Once you have seen firsthand how these mushrooms grow, you will naturally begin to see other avenues for exploration and experimentation.We have provided a list of titles at the end of the book for further reading, should you want to go beyond the boundaries of its pages, and we sincerely hope you will do so.To have outgrown our methods is to have proven their value as tools for learning.
Scope and Scale
Another thing you might notice on reading this book is that it does not contain methods for cultivating mushrooms on a larger scale, so-called “bulk” methods. After some deliberation, we decided not to cover the subject of large-scale cultivation for two reasons. First of all, bulk methods are far less reliable than the small-scale ones we describe here, particularly for beginners. Second, we felt that to do so would be to encourage unnecessary risk-taking.The methods we describe here should provide any reader with more than enough psilocybin to keep one’s friends and family “bemushroomed” for years. If you find you have more than you need, we encourage you to (discreetly) give them away, rather than sell them on the open market. Besides, while growing or possessing these mushrooms in any quantity is illegal in most countries, growing them in bulk and/or selling them is just asking for trouble.The small-scale methods we describe are far more suited to anyone trying to keep a low profile, and the best way to avoid being busted is to keep out of “the biz” in the first place.
If, after having succeeded with our methods, you still feel the urge to “bulk up,” we suggest you consider learning how to grow edible or medicinal mushrooms.You might not make quite as much money doing so, but you’ll certainly keep yourself out of jail. Methods for growing mushrooms of every kind (including the Psilocybes) on both small and large scales can be found in several of the books in our further reading list.
How to Read This Book
We strongly suggest you read this book from cover to cover, front to back, one chapter at a time, in the order presented. However, if like us you have a terminally short attention span, then feel free to skip around and read the book in whatever order you like. Just make sure that at the end of the day you have read the book in its entirety before you attempt any of the experiments within, even before you start gathering your equipment and materials, for two important reasons. First of all, mushroom cultivation is a complicated and strange process, and is not the kind of work for which everyone is necessarily well suited. It is entirely possible that upon reading this book you will find that you don’t really have the time or wherewithal to make a go of it. That is fine. Better that you figure that out before you invest any further time and money in the endeavor. Of course, the last thing we want to do is discourage you from trying. We truly believe that the methods we present are simple enough for just about anyone to perform successfully.We just want to make sure you really know what you are in for should you choose to give them a try.
Second, and perhaps more important, if you take the time to internalize as many of the ideas and processes we present as possible before beginning, you will succeed far more quickly than you otherwise would.We figured out this part the hard way. It was not until we had read every book we could find on the subject over and over and over again, and really felt like we understood what was supposed to happen, that things actually happened the way they were supposed to. In other words, it was only when we could see with our mind’s eye what we were supposed to see in the real world, that our experiments at last began to bear fruit, so to speak.We hope that this book is presented in such a way that upon reading it, you will understand what you will be doing and why, and you will experience swift success.
1
A BRIEF HISTORY OF PSILOCYBIN MUSHROOM CULTIVATION2
At the time of R. Gordon Wasson’s “rediscovery” of the shamanic use of psilocybin-containing mushrooms in Mexico in the 1950s, the science of mushroom cultivation was still very much in its infancy. Until then, the only species of mushroom under cultivation, at least in the West3, was Agaricus bisporus, the common white button mushroom. The cultivation methods used were more or less the same as those devised in France during the 17th century: growers collected mycelium-rich soil from wild areas where the mushroom was found and transferred it to rows of horse manure in naturally climate-controlled caves. This method was effective, but since it utilized a raw, unpasteurized substrate, it left much to chance, and the beds often succumbed to contamination.3
These crude methods remained essentially unchanged until the 20th century, when a number of incremental improvements were discovered, eventually setting the stage for the successful cultivation of Psilocybe cubensis in the 1960s. In the late 18th century, the American mushroom grower and researcher William Falconer published a book entitled Mushrooms: How to Grow Them; a Practical Treatise on Mushroom Culture for Profit and Pleasure, which compiled recent discoveries in Agaricus cultivation, and included a chapter on the benefits of a “casing layer.” By placing a thin layer of soil on top of the compost beds prior to fruiting, growers discovered that their mushroom yields were improved considerably.
Several years after Falconer’s book was published, scientists working at the U.S. Department of Agriculture discovered that many of the contamination problems previously associated with mushroom production were eliminated by using horse manure that had been subjected to heat sterilization before being inoculated with Agaricus mycelium.This process created in essence what was the first pure mushroom “spawn.” Then, in 1930, while working at Pennsylvania State College (still today one of the leading centers of mushroom cultivation research), mycologist James W. Sinden found that sterilized wheat grain made an even more effective and robust spawn substrate. Whole grain would in time prove itself a nearly universal spawn medium and has remained the medium of choice for the cultivation of many species of mushrooms to this day.
In the late 1950s, the French mycologist Roger Heim was the first to successfully cultivate several Psilocybe species, using materials brought back from his travels with R. Gordon Wasson in Mexico. To determine optimal conditions for fruiting, he tested each species they collected on a variety of sterilized substrates. With Psilocybe cubensis, he found that the best fruitings occurred on cased, sterilized horse dung. However, because of the relative obscurity of Psilocybe mushrooms and their powerful effects, along with the fact that Heim’s writings were not translated into English for nearly twenty years, his work remained mostly unknown to the wider world.
The latter part of the 1960s saw the publication of a number of “underground” pamphlets and booklets describing the manufacture and cultivation of a variety of psychedelic drugs (many of them at that time still legal to possess), among them several species of Psilocybe mushrooms. However, the techniques they described were either crudely presented or far too technical for the average person to utilize with much success, and many of the books gave the impression that perhaps even the authors themselves had not put their own methods to the test.
It was not until the publication of two books in the late 1970s, O.T. Oss and O.N. Oeric’s Psilocybin: Magic Mushroom Grower’s Guide (1976) and Dr. Steven H. Pollock’s Magic Mushroom Cultivation (1977), that reliable techniques of Psilocybe mushroom cultivation became widely available. While the methods these books described were still fairly complicated for the layperson to master, they were well researched and clearly presented, and with a modest effort and perhaps a little luck, just about anyone could make them work. The two books covered similar material, but each took a slightly different approach to the subject, and both would prove influential on future developments of the art.
O.T. Oss and O.N. Oeric were pseudonyms of brothers Dennis and Terence McKenna. Their book was the outgrowth of their experiments with Psilocybe cubensis cultivation on sterilized rye berries using James Sinden’s grain spawn methods. As the brothers discovered, this species grew and fruited quite happily from rye, especially when a sterile casing layer à la Falconer was applied atop the colonized grain. In their method, spores were germinated on a sterile agar medium and the resulting mycelium was transferred onto sterilized rye grain in quart canning jars. The casing layer was added directly to the colonized jar cultures, and fruiting would commence several weeks later. The relative simplicity of their method, with its reliance upon the use of more or less readily available ingredients and tools, along with the book’s quirky aesthetics and psychedelic, sci-fi musings, served to give it wide appeal, and spread the mushroom and its message far and wide.
Pollock’s book was decidedly less whimsical than the McKenna’s and only remained in print for a brief time, but it was perhaps ultimately just as influential. In it, he described the results of his experiments on the cultivation of a wide array of active Psilocybe species on a variety of substrates. While he too found that P. cubensis fruited from a number of different cereal grains, he settled upon brown rice rather than rye as his preferred substrate, since it was cheap and widely available. This was a fortunate choice for two reasons. First of all, some twenty years later it would be determined that mushrooms grown on brown rice are among the most potent reported for this species, containing as much as 1% alkaloids by dried weight.4 More importantly, it would later inspire one of the great advances in simple Psilocybe mushroom cultivation methods, the “Psilocybe Fanaticus Technique.” Sadly, Pollock never lived to take credit for his legacy, as he was murdered under mysterious circumstances in his Texas home in 1983, at the age of 33.
Meanwhile, in autumn of 1972, students at the University of Washington, Seattle, discovered that the bark mulch used to landscape buildings and greens around campus was covered with a species of Psilocybe mushroom, Psilocybe stunzii. It was quickly determined that these mushrooms, nicknamed “Blue Ringers” for the brilliant colors they turned upon handling, were quite active, and they soon became a popular recreational psychedelic. Though the mushrooms fruited rather prolifically on their own, observant students discovered that portions of mycelium-impregnated mulch could be transferred onto virgin bark to speed the dispersal of the organism and promote larger fruitings, much as had been done with Agaricus in France for hundreds of years. The intervening years saw the description of several Psilocybe species from the Pacific Northwest that were new to science, among them Psilocybe cyanescens, P. cyanofibrillosa, and P. azurescens. All of these discoveries led to the development of methods for the outdoor cultivation of wood-inhabiting Psilocybe species, as detailed in Paul Stamets’ (himself a student in Washington at the time) book, Growing Gourmet and Medicinal Mushrooms.
In 1991, an enterprising experimenter by the dubious name of Psilocybe Fanaticus published a new cultivation manual, The Psilocybe Fanaticus Technique. His book described a highly efficient and nearly foolproof technique of Psilocybe cubensis cultivation on brown rice and vermiculite “cakes” in half-pint mason jars. While this method (the “PF Tek,” as it came to be known) obviously borrowed much from its predecessors, it was unique in a number of important ways.
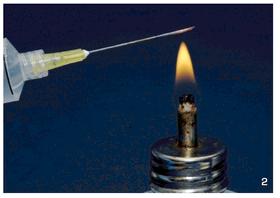
First of all, the substrate it utilized was a mixture of moistened brown rice flour and vermiculite. Its open, airy structure made it an ideal medium for the rapid and vigorous growth of the fungus, eliminating the need for shaking or otherwise disturbing the substrate after inoculation. It was also readily sterilized in a simple boiling water bath, obviating the need for one of the more prohibitively expensive and hard-to-obtain pieces of equipment previously essential for mushroom cultivation, the pressure cooker. Second, the PF substrate was covered in a thin layer of pure, dry vermiculite, which served as an effective barrier to contaminants during inoculation and incubation.This allowed the cultures to be handled openly without the need for glove boxes or careful sterile techniques. Minimizing much of the risk of contamination in this way did away with yet another obstacle that had previously stymied many a would-be cultivator. By utilizing an aqueous suspension of spores as inoculum, the PF Tek also eliminated the need for difficult and contamination-prone agar techniques. After the substrate had been sterilized, it was injected at several locations from a syringe containing a sterile spore solution. The pre-hydrated spores soon germinated at many locations throughout the jar, and the substrate quickly colonized.
Rather than relying upon a casing layer to promote fruiting, the PF substrate was popped out of the jar as a solid “cake,” which was then placed into a small chamber containing a thick underlayer of moist perlite (an inert water-absorbing material used in horticulture), which served to wick water into the cake as well as humidify the atmosphere within the chamber. When placed beneath sufficient lighting, the cakes soon fruited at many locations on their outer surfaces.
The utter simplicity of the Psilocybe Fanaticus Technique, combined with the rapid dissemination of information in the age of Internet news-groups and websites, created a flurry of new interest in Psilocybe mushroom cultivation and spawned an entire generation of amateur growers.5
Meanwhile, at the very same time that Psilocybe Fanaticus was perfecting his methods, another innovative amateur mycologist, Rush Wayne, PhD, was quietly preparing a cultivation revolution of his own. Wayne, a biochemist by training, had become interested in the idea of growing edible mushrooms at home, but his familiarity with the complications of sterile culture work had discouraged him from trying. That is, until he read a journal article describing the use of hydrogen peroxide (H2O2) in orchid seed germination. Apparently the peroxide killed bacteria, yeasts, and fungal spores in the agar medium, while leaving the orchid seeds themselves unharmed, since orchids, like most multi-celled organisms, produce peroxidases, enzymes that catalize the oxidation of compounds by peroxides. Wayne wondered whether this method could be applied to mushroom culture work, given that mushroom-producing fungi also synthesize peroxidases.
He performed a long series of experiments on different fungi and media, using a variety of peroxide concentrations, and discovered that his hunch was correct: most mushroom species grew quite happily in the presence of hydrogen peroxide, while contaminant organisms did not. As long as the media were sterile to begin with, the presence of relatively low concentrations of peroxide rendered the cultures resistant to contamination for long periods, allowing them to be handled in the open air without specialized techniques or equipment. As with the PF Tek, gone was the need for air filtration, clean rooms, or glove boxes. Wayne published the results of his research in a 1996 book, Growing Mushrooms the Easy Way: Home Mushroom Cultivation with Hydrogen Peroxide.
Hydrogen peroxide is ubiquitous in nature, thus it is not surprising that fungi should thrive in its presence. Chemically, it is simply water containing an additional oxygen atom. Since this makes it a relatively unstable molecule, the extra atom is readily released as a free radical. Free radicals are highly reactive and quickly bond to nearby molecules, which can themselves then become free radicals, beginning a chain reaction. If this cascade takes place unchecked within a biological system, it generally leads to cell death. Most multi-celled organisms, fungi among them, produce hydrogen peroxide and peroxidase enzymes as a means of protection against bacteria, yeasts, and viruses. In addition, fungi use peroxides and peroxidases to break down the cell walls of their food sources. Since most fungi produce peroxidases, hydrogen peroxide offers no protection against living fungi, including contaminant molds. Nevertheless it does destroy spores. Therefore, as Wayne discovered, as long as the medium was thoroughly sterilized or pasteurized to begin with, the addition of peroxide to cultures effectively protected them from all airborne contaminants.
Without question,Wayne’s discovery represented a true revolution for generalized mushroom cultivation techniques. What the PF Tek did for Psilocybe cubensis cultivation, the “peroxide tek” does for the cultivation of nearly all species of mushroom-producing fungi. A practice that had one been open only to experts with specialized skills and expensive equipment was now made available to anyone with a pressure cooker6, a few mason jars, and a clean-enough kitchen counter.
It is no exaggeration to state that the book you hold in your hands would not have been written without Rush Wayne’s discovery and writings. The inclusion of peroxide into our own repertoire allowed us to explore mushroom cultivation to a far greater depth than we had previously done. Chances are, without Wayne’s work, we would have called it quits in frustration long before we had even thought about writing our own cultivation manual. For that reason, our book is dedicated to Wayne, as well as to the many other pioneers of mushroom cultivation who preceded him. It is our wish that this book may similarly serve to motivate others to explore the fascinating and beautiful mysteries of the mycological universe.
2
THE BIOLOGY OF MUSHROOMS
Picture this: a cow patty on a summer day in a grassy field on a dairy farm somewhere in the sunny tropics. Atop and embedded within this cow patty stands a solitary, majestic specimen of Psilocybe cubensis. Its stem is sturdy and plumb straight, its cap open, flat as a dinner plate, shadowing the turd in its wide, dark penumbra. For all the world it looks like the cow patty has somehow acquired a parasol in order to shade itself from the ravaging effects of the sun’s rays. From out of the darkness rains a silent, invisible, seemingly endless cloud of spores, carried away to places unknown on each passing breeze. Hold this image in your mind as you read this chapter; in it you will find most of what you will need to know about mushroom biology.
This chapter contains a fair amount of complex information and technical jargon.You may find yourself yawning at the mere thought of wading through information about the behavior and biology of fungi. Then again, maybe you take great pleasure in exploring new areas of scientific knowledge. However you feel about the prospect, we ask you to bear with us, since understanding the underlying processes at play in the mushroom life cycle will make the cultivation techniques we present much clearer. If and when something goes not quite according to plan, this information will help you make real sense of what you are seeing so you can alter your approach appropriately.
This chapter is as much about dispelling misconceptions as it is about presenting new information. That’s because most of us think we have an idea about what mushrooms are and how they behave in the world, and most of these beliefs are quite mistaken.We know this from personal experience. When we first attempted to cultivate mushrooms, we assumed we knew all there was to know about them, and our efforts failed rather spectacularly. Only when we really began to understand their mysteries did we meet with success.
What is a Mushroom?
Relatively few of us have anything to do with fungi, at least not by choice.7 This is a cultural phenomenon as much as anything else.When most people think of mushrooms, they imagine either the bland and innocuous toppings on their pizza, or the exotic, ornate toadstools of fairy tale and legend, the mere taste of which will drive one mad, if not kill him outright. For the vast majority of North Americans, mushrooms are either fearful poisons or inoffensive vegetables, and in either case not worthy of much thought. Even if you are in that tiny minority for whom mushrooms do offer fascination, wonder, and delight (likely owing to one or more experiences with a species of Psilocybe), you probably learned little to nothing about them in your high school or college biology courses.
So what exactly is a mushroom? A mushroom is only one part of a fungus, and not a thing in itself, much like you and your left elbow are connected but can hardly be said to be one and the same. Strictly speaking, mushrooms are the reproductive structures of some fungi8, roughly equivalent to the flowers on an apple tree, which contains the “seeds” of future trees.
That said, fungi are neither plant nor animal, though they have similarities with both. Not surprisingly, there has always been a lot of confusion swirling around the proper classification of these mysterious and secretive creatures. Most of us tend to think of mushrooms and fungi as a strange variety of plant, since they often spring up from the ground like plants, and appear unable to get up and walk (or dance or swim) around like we lucky animals can. This is the primary misconception most of us have about fungi, and the one that you that you should dispense with straight away. So here it is: fungi are not plants, and growing mushrooms is not like gardening.9
Then again, fungi are not animals either, though despite appearances they are much more closely related to animals than plants. Plants, algae, and some bacteria synthesize their own food from sunlight, carbon dioxide, and water, and thus are known as autotrophs. All other organisms, fungi included, are heterotrophs, meaning they derive energy from plants, or things that eat plants (say, a fish), or things that eat things that eat plants (a bigger fish).That’s pretty much where the similarities between animals and fungi end, however.
Fungal Classification & Taxonomy
In order to understand how fungi fit into the “animal, vegetable, mineral” order of things, you need to understand the more formal system biology uses to classify organisms, which is known as Linnaean taxonomy (named for Carolus Linnaeus, the 18th century Swedish botanist and physician who first devised it). In this system, every individual species is given a unique two-part (or binomial) Latin name, such as Psilocybe cubensis or Homo sapiens.These two names refer to the last two categories, Genus and Species, of an eight-part hierarchy that organizes all living things by their biological similarity to one another.10 The divisions, in order from largest to smallest, are Domain, Kingdom, Phylum, Class, Order, Family, Genus, and Species. The easiest way to get a sense of this system is to see it in action:
Examples of the Linnaean Taxonomic System
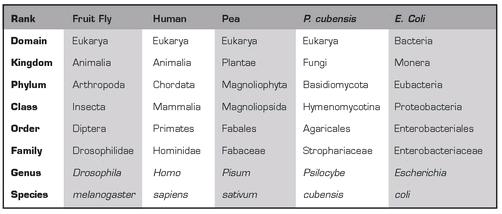
As you can see from the table, the higher you go in the ranking, the greater the number of species included in each category. Fruit flies, peas, humans, and Psilocybe mushrooms are all found in the same domain, Eukarya, and thus are all more closely related to one another than they are to bacteria. Conversely, the further down in the rankings that you go, the more species begin to diverge from one another.
For our purposes here, the most important ranks to consider are Kingdom, Genus, and Species. There are five kingdoms11, and the fungi reside within their own, the Kingdom Fungi. While there are numerous variations on the theme, the one thing all fungi have in common and what sets them apart from other organisms is that they digest their food externally and then absorb its component nutrients into their cells. All species of fungi described in this book are in the genus Psilocybe. Finally, every species has a unique binomial, such as Psilocybe cubensis or Psilocybe azurescens.12
The Fungal Life Cycle
In order to get a good sense of exactly what fungi are, it helps to understand what they do for a living, how they get around, and what kind of love lives they lead. A good way to get a handle on this is to trace the fungal life cycle, the journey from birth to death, repeated endlessly with each successive generation. Understanding the life cycles of organisms is an excellent way of sorting out what is unique to each of them, since no two species do it quite the same way.
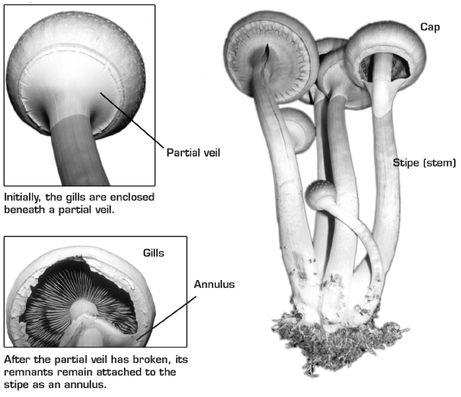
Sexual reproduction is the recombination of the genetic material from two parent individuals to form a new one. The container of genetic material donated by each parent is known as a gamete. The gametes of fungi are called spores. A spore is a compact, protected cell, capable of remaining alive but dormant for long periods of time until it finds a suitable home. All of the fungi we will discuss in this book are known as Basidiomycetes, since they produce their spores on basidia, tiny baseball-bat-shaped protuberances lining their gills, the blade-like structures arranged in a radial pattern on the underside of the cap, or pileus.13 The pileus is held aloft on the end of a cylindrical stem, known to mycologists as a stipe.
Parts of the Mushroom
Spore Discharge
Let’s return to our cow patty and its lonely mushroom. Zoom in closer: deep in shadow, millions of microscopic, baseball-bat-shaped basidia stick out from the flat faces of the gills lining the underside of the parasol, and at the wide end of each basidium stand four ovoid, purple-black spores. Each spore is perched like a top upon a tiny horn-shaped protuberance at the outer end of the basidium, known as a sterigma. The air around the gills is moist and much cooler than that around the mushroom, thanks to the wonders of evaporative cooling taking place on the sun-beaten upper face of the cap. As the air cools, water condenses around the spore and its tiny stand, and a droplet begins to form at the place they join. The droplet grows until it can no longer support its own structure, its surface tension breaks, and the water from the droplet spreads out over the body of the spore. The force of this action draws the spore toward the sterigma. The sterigma, being somewhat elastic, collapses slightly beneath the weight of the spore, only to push back with an equal and opposite force and catapult the spore from its perch into the open space beyond the face of the gill.14 The amount of force is precisely calculated to hurtle the spore far enough to clear the surface of its own gill, but not so far that it smacks into the facing one. Instead, it succumbs to gravity and is pulled straight down and out below the bottom face of the mushroom, where with a little luck, it will be carried away by a gust of wind, along with millions of its siblings.
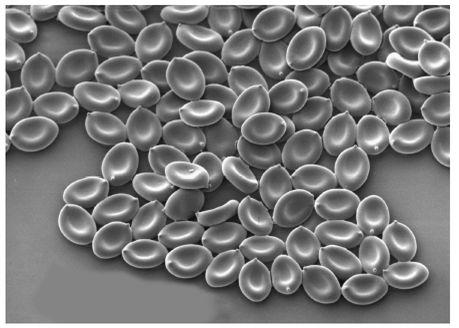
An electron scanning micrograph of
Psilocybe cubensis
spores.
When the wind in our field subsides, two spores from our mushroom have settled onto a patch of grass, where they now wait patiently for something or someone to bring them closer together.
Fungal Growth
Now picture a cow, maybe the one who made that same cow patty from the beginning of the chapter. The cow is munching on the grass in our field, because that’s what cows like to do, and sooner or later, she eats the blades of grass upon which sit our lonely spores, munching them down with her lunch. Swallowed whole with the grass, they are swept through her digestive tract only to emerge some time later at the other end. Fortunately, the spores are resilient and well armored, and suffer no ill effects from their wild ride through the cow’s guts. Better than that, for their troubles they find themselves smack in the middle of a pile of their favorite food: cow shit.15 Soon afterward, each of our spores germinates, its cells dividing and slowly growing out into the delectable and nutrient-rich materials in the cow patty.
Growing fungi consist of networks of hyphae: tubular, filamentous cells that expand and divide at their forward tips, branching occasionally to create fork- or fan-like structures. Masses of hyphae are known collectively as the mycelium of the fungus. To the naked eye, fungal mycelium appears often as white, fuzzy or hair-like growth on the surface of the food source (or substrate), such as you might see on the underside of an upturned log. Most fungi spend the majority of their days as an undifferentiated mycelium, only occasionally forming specialized, complex structures such as mushrooms.
Hyphal growth is also invasive, meaning it occurs within and often throughout the substrate. Digestive enzymes secreted from the tips of the advancing mycelium into their surroundings degrade the substrate into simpler organic molecules, to be absorbed or engulfed by the mycelium as it marches along. In effect, fungi do their digesting on the outside. While we tend to process our meals in the privacy of our own insides, fungi prefer to eat out.
All of the fungi we discuss in this book are saprophytes, or saprobes, meaning that they derive their nutrition from non-living organic matter, in this case dead or decaying plants. This is in contrast to parasitic fungi, which colonize and digest living organisms, often killing their host in the end, and mycorrhizal fungi, which live in a symbiotic relationship with their plant hosts.16
Fungal Sex, Part One: Mating
So our spores, now grown into two individual mycelial colonies, continue to explore the cow pie, slowly penetrating and absorbing its contents, while blindly reaching out for one another. Eventually, their colonies of mycelium touch, and at last our two lovers meet. However, all of their good fortune thus far is no guarantee they will decide to tie the knot, since fungi are just as picky as we humans when it comes to whom they choose as mates. In order to minimize inbreeding and to promote genetic diversity, fungi produce spores of multiple mating types. Mating types are roughly equivalent to our two sexes, except that with fungi the number of different “genders” can be anywhere from two to many thousands!17 In order for two strains of monokaryotic fungi to mate, they must be of different mating types. Fortunately for our lovers (and for our story) they are quite compatible, and it is nothing less than love at first sight.
Life Cycle of
Psilocybe cubensis
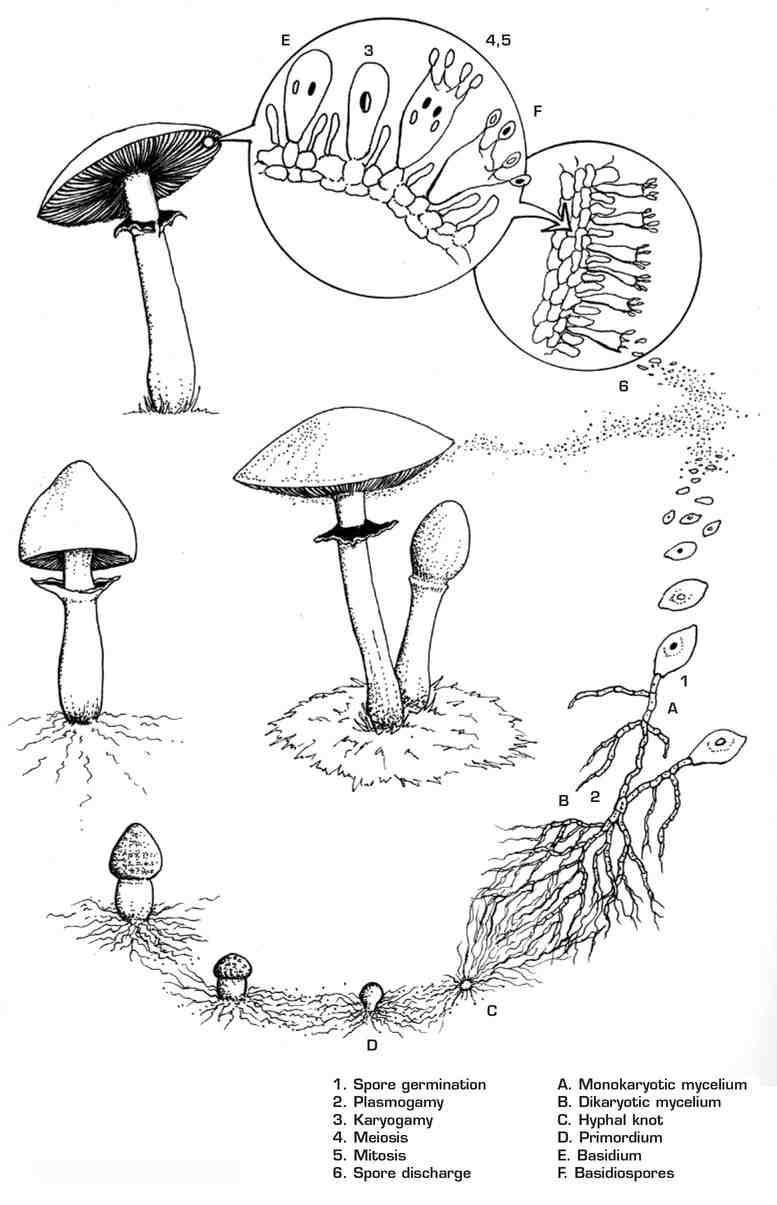
Up to this point, the cells of each individual mycelium have been monokaryotic: their cells contain but one haploid nucleus, with only half the genetic material of a mature fungus. Monokaryotic mycelium, being immature, is thin and wispy in appearance, and slow growing. When the two colonies fuse, they produce a mycelium composed of cells containing two nuclei, known as dikaryotic mycelium. Dikaryotization is a state unique to most Basidiomycetes, where the cells from two compatible gametes join together into one cell type, but their individual nuclei remain separate. Unlike the cells in your body and those in most other higher organisms, each of which contain a single diploid18 nucleus, Basidiomycetes live most of their days with two nuclei per cell, one from each “parent” monokaryotic mycelium. The only time Basidiomycetes combine all of their genetic material into a diploid nucleus is during a single, brief moment inside of each basidium, just before spores are generated. In a sense, these fungi start the sex act near the very beginning of life, only to finish it much later, living their lives in what amounts to a continuous act of foreplay.
Finally, our two lovers are combined together into a single organism, a mature fungus, and now that our fungus is mature, it can do what mature fungi love to do: eat. The fungus invades the substrate of the cow patty with dense, ropy strands of mycelium. It will continue to do so until the food source is exhausted of available nutrition, or some other environmental shift induces it to produce mushrooms, or fruit.
Fungal Sex, Part Two: Fruiting
All that remains now to bring us back full circle to where we began is for our fungus to produce mushrooms, bearing a new generation of spores. Exactly why and when fungi decide to form mushrooms remains somewhat of a mystery, and reasons vary greatly among species. Some do so because of a change in the weather, such as a heavy rain, an increase or decrease in temperature, or both in combination. Others produce fruit only after the substrate has been fully colonized and its available nutrients exhausted. In all of these cases, the fungus is likely provoked into reproducing by the increasing probability of its own demise. Still other species wait years to fruit, only doing so after some subtle environmental change has occurred. Fortunately for us, Psilocybe cubensis is a promiscuous species and does not need much encouragement. Robust P. cubensis strains will fruit readily and abundantly under a wide variety of environmental conditions.
Many fungi, Psilocybes included, want to ensure the vertical orientation of their caps in order to maximize the elevation and efficiency of spore release. For this reason, they fruit at the upper surfaces of the substrate, using sunlight as a trigger. Once the mycelium of our fungus has reached the upper layers of the cow patty, tiny knots of intertwined hyphae form at numerous places on its exposed surface. Soon thereafter, these hyphal knots develop into primordia (singular, primordium), also referred to as pins or pinheads: miniaturized, complete versions of the full-sized mushrooms they will eventually become. It is at the pinning stage that the fungus first begins to truly differentiate and form a variety of unique cell types. The upper surfaces of the tiny caps darken, while inside the primordium the cells that will comprise the cap, stipe, gills, and veil divide and orient themselves appropriately. Their nuclei divide and accumulate, while walls (or septa) form around them, creating a dense matrix of compacted cells. A mature primordium contains all of the cells that will be present in the fully-grown mushroom; all that remains for it to do is to take up water and expand.When it does, it happens rapidly, literally exploding into being.19

The annulus of this
P. cubensis
fruitbody is coated with a dark deposit of spores.
In a rapid surge of growth, the familiar features of the mushroom begin to take shape.The stipe elongates, the spherical cap expands and then begins to flatten, exposing the partial veil, a thin membrane that serves to protect the fragile, developing gills. When the gills are fully formed, the cap expands. This causes the veil to pull away from the outside of the cap.Veil remnants often remain attached to the stem, hanging loosely like a tiny skirt, known collectively as an annulus.
The elongating mushrooms use light, air currents, and gravity to orient their caps as vertically as possible, ensuring optimal spore release once the gills open. On the vertical faces of the gills, in a dense layer of cells known as the hymenium, millions of basidia are forming. Once the basidium reaches maturity, its haploid nuclei fuse to form a single diploid nucleus, and the sex act that began when our two spores first met is finally complete.
This phase is short-lived, however, since this nucleus rapidly divides, shuffling its contents to form four genetically unique, daughter haploid nuclei. These nuclei migrate into the stergimata, where they are encased and deposited as spores at the end of the basidium.There they wait for their moment to fly, and for our story to begin once again.
The Biology of Mushroom Cultivation
Hopefully by now you have a sense of how most Basidiomycete fungi behave in a natural setting, and should have no trouble understanding how such mushrooms are artificially grown.While the context has changed, the biology remains the same. Nature leaves much up to chance, improving her likelihood of success by virtue of great numbers: many fruits, millions of spores per fruit, and perhaps hundreds or thousands of strains per generation, some small percentage of which will thrive. The cultivator, on the other hand, succeeds at each stage of the process by carefully selecting only the best candidates for further advancement, and by working within a controlled (sterile) environment.
Mushroom cultivation proceeds through three basic phases, regardless of the species of fungus: germination or isolation, expansion, and finally fruiting. The first stage involves isolating a mushroom culture from spores or from the tissue of a living mushroom. Spores germinated on nutrified agar in Petri dishes result (after mating) in a diversity of strains within the same culture, while tissue culture results in a genetically identical clone of the parent mushroom. In either case, the growth of the fungus in the medium gives rise to a dikaryotic mycelium.The use of a semi-solid agar medium allows the cultivator to easily examine the culture for desired characteristics and to identify contamination, if present. The mycelium can be propagated on agar more or less indefinitely and can be stored at this stage at cold temperatures for later retrieval.
Once a suitable clean culture has been isolated, the mycelium is then transferred to a secondary medium, usually sterilized whole grain in quart-sized mason jars. The purpose of this stage is to expand the volume of mycelium (the mycelial mass) to an amount that will support the desired amount of fruiting in the final phase. A small amount of mycelium on a wedge of agar is removed from a plate and placed on the grain.The mycelium grows from the agar onto the grain.The jars of grain are shaken every few days to facilitate colonization. When the grain is fully colonized, they are then used to inoculate larger containers of grain (usually in sterilizable plastic bags, though larger mason jars will also work), to further expand the mycelial mass. The material generated in this phase is generally known as spawn.
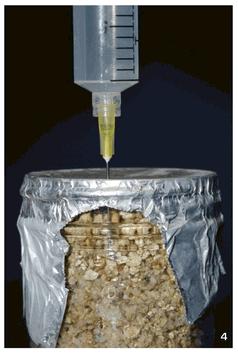
Once a suitable amount of spawn has been generated, it is used to inoculate a final substrate, otherwise known as the fruiting substrate.The exact constituents of the fruiting substrate depend on the species in question. Some species will fruit from a variety of substrates, while others are much more particular. Psilocybe cubensis, for example, will fruit from wheat straw, cow manure, or even from grain itself, while Psilocybe azurescens and other wood-loving species will fruit only from a bed of hardwood chips. Once a suitable medium has been prepared (sterilization is usually unnecessary at this stage, though sometimes the substrate is pasteurized), it is mixed with spawn and left to colonize. After the fruiting substrate is colonized, fruiting is initiated. Once again, conditions for initiation are species specific, but generally involve the covering of the substrate with a layer of moisture-retaining, non-nutritive material such as peat moss (known as a casing layer) and/or modifying the temperature, humidity, air exchange, and light levels at the fruiting surface to create conditions that favor mushroom formation. Eventually, if all goes according to plan, mushrooms form, first appearing as primordia, then enlarging to full size within a few days, at which point they begin to release their spores, and the cultivation cycle is complete.
Cultivation Flowchart
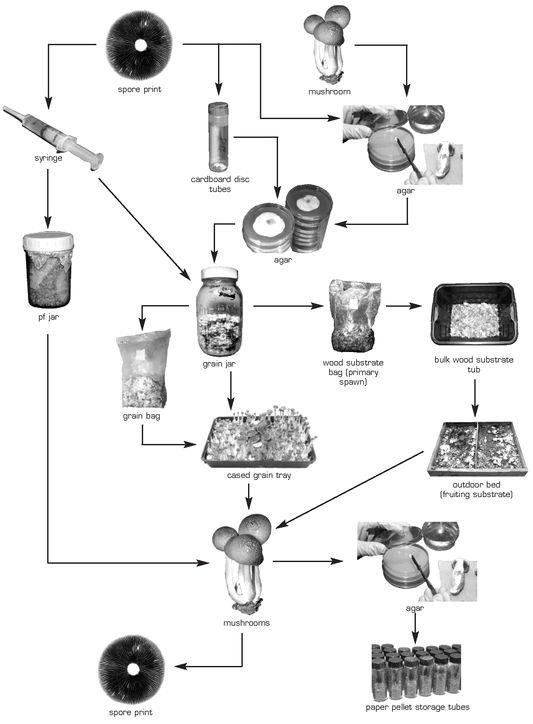
3
PSILOCYBE: THE SPECIES
There are some thirty thousand documented species of mushroom-producing fungi worldwide. Of these, approximately one hundred species or varieties are known to contain psilocybin or related compounds. Most of these are found within the genera Psilocybe and Panaeolus, with a few appearing elsewhere in Inocybe, Conocybe, Gymnopilus, and others. Of course, not every species in these genera contain psilocybin, and even those that do may only produce it in trace amounts.
In this book, we present methods for the cultivation of two types of psilocybin mushrooms: the coprophilic (or dung-inhabiting) species Psilocybe cubensis, and the complex of interrelated lignicolous (wood-inhabiting) species such as Psilocybe azurescens and P. cyanescens. We chose to focus on these particular species for several important reasons: they produce psilocybin in relatively high quantities, they have a long history of cultivation, and they fruit reliably under easily reproducible conditions. In addition, they offer the possibilities of indoor (with P. cubensis) and outdoor cultivation (with any of the species in the P. azurescens complex). While there are certainly other well-known species that also meet these criteria, the two types we have chosen should produce ample quantities of psilocybin for any diligent grower.
The aim of this chapter is to familiarize you with these species, including their natural habitat, distribution, and behavior, so that you understand their basic biology as you begin work with them. This book is not meant to be a “field guide” and does not prepare you to find and collect these species from the wild. Foraging for mushrooms, whether for food or for psilocybin, requires a great deal of knowledge and skill. Being poisoned as a result of misidentification is a real and potentially lethal risk. If you are interested in collecting your own mushrooms, we suggest you closely familiarize yourself with at least several good field guides (we have listed several excellent guides to North American fungi in appendix C) and consult directly with experts who already know the fungi of your area. Chances are you have a local mycological society or club where there are people who can teach you what you need to know in order to identify mushrooms from the wild.
For further reading about the many psilocybin-containing mushrooms found worldwide, Paul Stamets’ Psilocybin Mushrooms of the World is currently the most comprehensive text on the subject, and is an essential addition to any mycology library.
Psilocybe cubensis
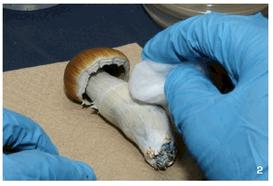
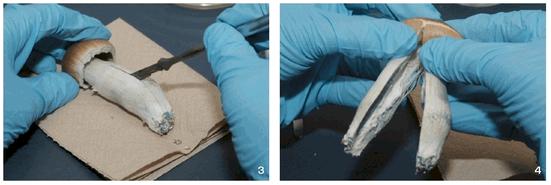
Psilocybe cubensis is the most widely cultivated species of psychoactive mushrooms, for both historical and biological reasons. Worldwide, it is one of the most common psilocybin containing species found in the wild, and therefore among the most commonly consumed and most well known. It is also one of the easiest to cultivate, since it fruits on a wide range of substrates, and under a variety of environmental conditions. Though in the wild it grows exclusively on dung, under cultivation it will fruit from just about any substrate sufficiently high in carbon and nitrogen: cereal straws, grains, grasses, corn, even from wood, paper, or cardboard, if supplemented with some form of protein. Most mushroom species are quite finicky in their growth and fruiting requirements, but not P. cubensis. This fact, combined with its ample potency, makes it one of the best species for the novice cultivator to grow.

Psilocybe cubensis
fruiting from a tray of cased wheat berries.
We begin with Psilocybe cubensis because it is both the easiest to grow and the species of psilocybin-containing mushroom with which people are most familiar. Its fast growing, deeply rhizomorphic mycelia, abundant primordia, large, robust fruits, and prolific spore production combine to make it among the most prototypical of Basidiomycetes. Once you have worked with P. cubensis for a while and have grown familiar with the mushroom life cycle, you will be ready to work with species that behave in more subtle ways.
Psilocybe cubensis is a pan-tropical mushroom that grows abundantly on the dung of cattle, horses, and elephants, or on soils containing their manure. It can be found almost anywhere in the world with a wet, warm climate, including Southeast Asia and Australia, India, Mexico, Central America, northern South America, and the Caribbean. In the United States, it is commonly found in the southeastern U.S. in the late spring and early summer, from Florida to the Texas Gulf Coast.
It is among the largest of psilocybin-containing species, with caps from ½ to 5 inches across, and thick stems up to 8 inches long.When grown on grain or rice, it is usually modestly sized, but on manure or compost it can produce enormous, hefty fruits. It produces dark, dense purple-brown spore prints.
When handled, Psilocybe cubensis often bruises deeply blue. Although a bluing reaction often indicates the presence of psilocybin in a mushroom, such evidence by itself cannot be considered definitive proof, since there are other unrelated fungal compounds that behave similarly. In addition, the absence of the bluing reaction does not necessarily rule out the presence of psilocybin-like molecules in a mushroom.The bluing reaction occurs when psilocin oxidizes into an as-yet uncharacterized dark blue chemical. Mushrooms containing low levels of psilocin, but significant levels of psilocybin, will not turn blue, despite their activity. (See page 52 for a picture of the bluing reaction in Psilocybe cubensis.)
P. cubensis is considered moderately potent compared to other active species. It can contain up to 1.2% (dry weight) of psilocybin, psilocin, and baeocystin, with the average somewhere around 0.5%, or 0.5mg/gram. While such averages are useful benchmarks for the comparison of the potency of one species to another, it is important to keep in mind that potency can vary widely among mushrooms of the same species. Certain strains, or the same strain grown under differing conditions or on different substrates, can display drastic variation in potency. Even the same culture can vary from one flush to another, with the second and third flushes usually being the most potent.
The Woodloving Psilocybes
Though Psilocybe cubensis is very easy to grow, there is one type of home cultivation to which it is poorly suited: the outdoors. In the wild, of course, it grows outdoors, and it certainly can be cultivated in a garden or wooded setting, but there is no real advantage to doing so. The two main benefits of establishing an outdoor mushroom garden is that it can be both perennial and clandestine. You set it up in an out-of-the-way place, forget about it until it fruits, harvest the mushrooms, and then forget about it all over again, until the process repeats itself the following year. Once established, a secret mushroom patch should be more or less self-sustaining and completely inconspicuous except when fruiting.
Psilocybe cubensis doesn’t fit the bill for this kind of set-up, for a number of reasons. First of all, it fruits rapidly and continuously until its substrate is exhausted of nutrients, and doesn’t linger long enough to be considered perennial. Second, it grows on and fruits from a wide variety of substrates, but so do a whole host of other undesired organisms. Unless the fruiting substrate is kept sterile (or at least extraordinarily clean), it will be colonized by molds and bacteria long before the mushroom can become fully established.That is why it is almost always grown indoors under very carefully controlled conditions. Finally, being a tropical species, it does not grow well in cooler climates and certainly cannot survive the below-freezing temperatures common in many places during the winter months.
Fortunately for the would-be mushroom gardener, there are a number of other Psilocybes that are up to the task. These are the lignicolous, or wood-loving, species, a group of related psilocybin-containing mushrooms that grow on wood chips or bark mulch and, owing to their appearance, are collectively known as the “caramel-capped” Psilocybes. This group encompasses as many as 10 species including Psilocybe cyanescens, P. azurescens, and P. cyanofibrillosa, which are all native to the Pacific Northwest of the United States, the Eastern European species P. serbica and P. bohemica , and P. subaeruginosa and P. tasmaniana, which are from Australia and New Zealand.
In addition to their similarity in habitat and appearance, these mushrooms all share another important characteristic: they are among the most potent of the known psilocybin-containing species. Of these, Psilocybe azurescens, with its species epithet alluding to the deep bluing reaction that occurs upon its handling, reigns supreme, containing up to 2.5% (dry weight) of psilocybin alkaloids, more than twice as much as that found in P. cubensis. Other woodlovers are somewhat more modest in potency, with reported maximum concentrations in the 1-2% range. Nevertheless, what the lignicolous species of Psilocybes lack in abundance and stature when compared to Psilocybe cubensis, they more than make up for in strength, and a relatively small garden bed of just one of them can easily provide enough psilocybin to last the gardener a long time, or at least until the next year’s fruiting.
While these species are all relatively similar in appearance, there are differences among them. However, under cultivation they all behave more or less identically, and the methods we provide later in the book will work for any of them. To better familiarize the reader with some of their characteristics, we have provided photographs and brief descriptions of the three species we have cultivated (see pp. 51-57). For complete details about this complex of species, you should consult Paul Stamets’ book, Psilocybin Mushrooms of the World.
Psilocybe cyanescens
Psilocybe cyanescens is a moderately potent species commonly found in the Pacific Northwest, from San Francisco to Canada. Its most distinctive feature is an undulating cap margin (the mycological term for the outer edge of the cap), which gives its mushrooms the nickname “wavy caps.” It grows on wood chips or woody debris in lawns, garden beds, and along mulched pathways. When young, its mushrooms have a prominent, cortinate (“web-like”) partial veil, which rapidly disintegrates at maturity. P. cyanescens has a relatively high psilocin content, and blues quickly when bruised.
Psilocybe azurescens
Psilocybe azurescens is the most potent known Psilocybe mushroom. It is similar to P. cyanescens in appearance, except that it lacks the latter species’ wavy margin, and often displays a pronounced nipple-like bump on the center of its cap, a feature known as an umbo. In the wild, it grows commonly on wood debris in sandy coastal soils, often under dune grasses. P. azurescens has a particularly high baeocystin content, which may account for its allegedly unique psychedelic “signature”; users commonly report that it produces a deep and strongly visionary effect, without significant associated physical discomfort.
Psilocybe cyanofibrillosa
Psilocybe cyanofibrillosa is a small wood-loving Psilocybe common to the Pacific coast of the U.S., from San Francisco to British Columbia. It is not considered particularly potent, containing only around 0.25% alkaloids by dried weight. However, there is evidence to suggest that a greater percentage of alkaloids are lost on drying this species than with others, making fresh P. cyanofibrillosa specimens more potent than would be expected.
Psilocybe bohemica
Psilocybe bohemica is a central European relative of the North American lignicolous Psilocybes, found in Germany, Austria, and the Czech Republic. It is similar in appearance to P. azurescens and P. cyanofibrillosa and slightly less potent than P. cyanescens, averaging around 1.1% alkaloids by dried weight.
Psilocybe subaeruginosa
Psilocybe subaeruginosa is a relative of P. cyanescens and P. azurescens that is native to Australia and Tasmania. It is similar in appearance to P. azurescens, though slightly smaller in stature, and its habitat is comparable to P. cyanescens. Chemical studies of this species are limited, but it is generally considered to be a moderate to highly potent species, as it bruises deeply blue on handling.
4
STERILE CULTURE TECHNIQUE
Food for mushrooms (properly known as its substrate) is much like food for humans: a nutritious mixture containing a balance of carbohydrates, protein, minerals, and vitamins. Also like our food, it is quite delectable to a variety of microorganisms, as a loaf of bread left out on the kitchen counter for more than a few days will quickly prove. However, unlike humans, fungi are microorganisms too, and have to compete for food with any other microorganisms in the neighborhood. Here bacteria and molds have the competitive edge, since they are able to reproduce thousands, even millions of times faster than the average mushroom species can. Any substrate containing even a single mold spore or bacterium is likely to end up a moldy or mushy mess.
In addition, the average cubic centimeter of air in the average room contains more than 100,000 particles. An invisible, silent rain of mold spores, dust particles, and pollen grains constantly settles upon every horizontal surface in your home, no matter how scrupulously clean you think it is.
The only way to keep these critters from hijacking your mushroom cultures is to make sure they never get onto them in the first place. There are two basic ways to do this: thoroughly kill whatever molds or bacteria are there to begin with, and exclude any others by working in a truly clean (i.e., sterile) environment. We eliminate contaminants from our materials by sterilizing, or autoclaving, them in a pressure cooker, where virtually no living thing can survive the high temperatures (121˚ C/ 255˚ F) and pressures (15 psi) within.We then create a sterile work environment by filtering the air in our workspace and/or sterilizing it with chemical disinfectants.
These two methods constitute sterile or aseptic culture technique, which is by far the most important thing you need to learn in order to succeed in mushroom cultivation. Let me reiterate this for emphasis: sterile culture technique is the most important thing you can learn from this book. If you don’t figure this one out, none of the cultivation methods will work, no matter how closely you follow the instructions. If you are really, really lucky, you might harvest a mushroom or two, but mostly you will have grown a dazzling array of blue, green, and black molds and a slimy, stinky collection of bacteria. Many would-be mushroom cultivators have failed right here, and those who have succeeded (your humble authors included) learned the hard way how and why to use sterile culture technique. It is our hope that the methods described in this chapter will show you the easy way, saving you a lot of time and heartache.20
So, one more time: sterile culture technique is really really really important.
Cleaning Your Work Area
The first task is preparing a clean workspace. Ideally, you can devote a room or space solely to your mushroom projects, such as a spare bedroom or an unused walk-in closet. If no such space is available, then much of the lab work can be completed in an average-sized kitchen, but this requires you to establish and maintain a pristine level of cleanliness. The kitchen competes with the bathroom for being the messiest and most biologically active room in the house, and mold counts tend to be very high there. On the other hand, working in a kitchen provides convenient access to a water source and a stovetop. If you plan to devote a separate space to mushroom work, make sure it’s close to the kitchen. There’s no point in sterilizing your materials only to carry them through a dirty house to your lab.
The workspace should have a good-sized table, preferably one with a continuous, easily cleaned upper surface. Formica or enamel is ideal, since you will need to wipe the workbench with alcohol before each use. If you have a wooden table, consider laying a piece of thin plywood with a plastic laminate surface or a piece of heavy, thick vinyl on top of it when you work. Similarly, the workspace floor should be easy to clean (linoleum or tile) and easy to inspect for cleanliness. Carpets are repositories of spores and dust, millions of which are kicked up into the air with each and every footstep, and should be avoided if at all possible. The walls should be clean (a fresh coat of paint wouldn’t hurt), and any other spaces and surfaces in the room should be thoroughly cleaned. Use a disinfecting solution if practical (orange-oil based products are good, since they are mild but effective biocides and environmentally benign). Obviously if you are working in your kitchen, you can’t disinfect every surface each time you plan to use it, but you should still give it a periodic deep cleaning and disinfect as much of it as you can before each use.
The space should be free of drafts to keep air movement around your cultures to a minimum. Windows should be closed tightly, heating or air conditioning ducts should be covered, and doors should be shut long before you begin your work.
Eliminate other sources of contamination from the room whenever possible. Potted plants, fish tanks, pets’ food dishes, litter boxes: get ’em all out of there.
Running an air-filtration device in the space is helpful too. Nowadays, good ones cost less than $100, and they are quiet and efficient enough to run continuously. Make sure the unit you buy is HEPA-rated. HEPA stands for High Efficiency Particulate Air. It is an official filter rating, which means that it captures particles 0.1 microns (1/100th of a millimeter) and larger, or 99.97% of the solid matter that is in the air.We keep our filter on low at all times, and run it on high for at least an hour before working in the lab to give the air in the room a thorough scrubbing.
Finally, you need to clean the air in your immediate work area.You can do this by working inside a glove box, an enclosed space that can be thoroughly disinfected and is draft-free, or in front of a flow hood, a large HEPA filter unit that blows a steady stream of pure sterile air over your workspace, excluding all contaminants. A glove box can be built easily and cheaply, but is less efficient, since air from the room can find its way inside. A flow hood costs considerably more, but is money well spent, since it allows you to work out in the open while still maintaining aseptic technique. Instructions for building both devices can be found in appendix B.
Personal Hygiene
Now that you have cleaned and prepared your space, it is time to consider the other primary source of contamination in your makeshift lab: you. Your body, hair, and clothes are an Amazon jungle of bacteria, viruses, and fungi, all invisible to your eyes and mostly harmless to you or others, but deadly to mushroom cultures. In order to keep this nasty horde to a minimum, you should be as clean as possible before each work session. This means showering, drying off with a freshly laundered towel, and dressing in a clean set of clothes immediately before working.
Your choice of clothing is important too; don’t wear long-sleeved shirts or loose fitting items that might flop around as you work. If you have long hair, tie it back on your head. Wipe your hands and lower arms with isopropyl (rubbing) alcohol and always wear disposable surgical gloves while you work (wipe the outside of the gloves with alcohol too).
Mental Hygiene
Just as you have prepared your workspace and your body, make sure you also attend to your state of mind before you work. Mental hygiene is as important as personal hygiene, since your state of mind will affect how you work, and if you are distracted or hurried, you will likely make mistakes or introduce contamination into your cultures. Your movements in the lab should be careful, measured, and deliberate. Avoid unnecessary fast or jerky motions, as they only create unwanted air currents. Take your time. If you are rushed, slow down, or save the project for a day when you have more time. Similarly, ask your spouse, children, dog, or cat not to enter the room or disturb you while you work and disconnect the phone. Play soothing, uplifting music if you like, but avoid Stockhausen or speed metal, unless you happen to find them relaxing to your ears.
Record Keeping
Keeping thorough and detailed records of all experiments is essential to any effective lab technique. Most steps in mushroom cultivation expand upon previous ones; for instance, each agar plate can be used to inoculate 10 new plates or 6 jars, each jar can inoculate 6 bags, and so on. It is easy to generate hundreds, even thousands of individual cultures. Having a way to identify each culture quickly and easily will save you loads of time and effort in the long run. It will also allow you to judge the progress of your work, helping you identify successes and failures and track each of them back to the source.
We mark each culture container with a coded numbering system, and record lab work and codes in a lab notebook. Any notebook will do, though lined or graph-ruled pages are helpful for separating data and making tables or diagrams. If the pages of the book are not already numbered, add page numbers at the top of each page.
Every time you create a new set of experiments (for simplicity’s sake, any individual culture container should be considered one experiment), start on a blank page. At the top of the page, write the date and a note about what experiments you performed (“MYA plates” or “rye grain jars”) including any pertinent information, such as specific recipes followed or unusual methods used. Under this, create three basic columns titled from left to right: the originating cultures, that day’s experiment numbers, and notes.
We use a series of hyphenated numbers to systematically label experiments. They follow the format: XX-YY, with XX being the current page number, and YY designating each individual experiment, in ascending order. In the column to the left of each number, we write the species name or an appropriate abbreviation, followed by a hyphen and a number referring to the specific strain in question (“PC-1” for Psilocybe cubensis, strain 1, “AZ-3” for Psilocybe azurescens, strain 3, for example). Below the species and strain code, we note the experiment number of the originating culture in parentheses (e.g., “(14-2)”). Since each originating culture is generally used to inoculate a number of new ones, each set of experiments can be grouped together by placing a solid line after the last new culture, and bracketed so that the original culture information only needs to be recorded once.
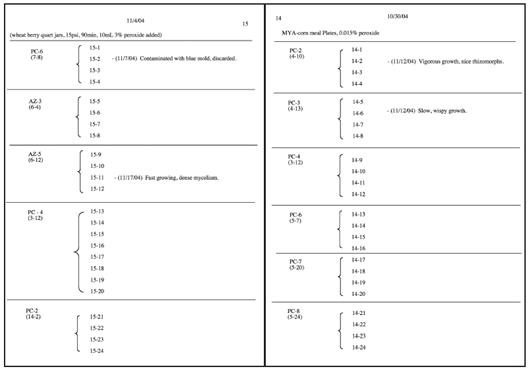
Two example pages from the record-keeping system.

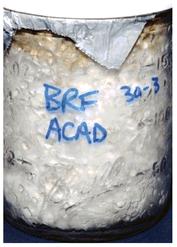
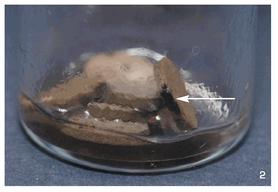
A grain jar culture of
P. subaeruginosa
marked appropriately using this record-keeping system.
The right-hand column is used to record notes about each culture as it grows out over time. Date each note, so elapsed time is tracked as well. Meanwhile, the actual cultures you generate are marked with corresponding labels using a permanent marker on the edge of the plate or the outside of the jar. This includes the species/strain code (e.g., “PC-2”), the originating experiment in parentheses (e.g., “(14-2)”), and the new experiment number (“15-21”).
It is easier to perform record keeping duties all at once, after the experiments have been completed for the day. Instead of writing in the notebook as you work, simply record the originating culture and strain code on the outside of the containers. Then when you are done with all of your experiments, fill in the notebook pages and add the new experiment numbers to the containers.
5
EQUIPMENT AND SUPPLIES
Mushroom cultivation requires equipment, including many specialized tools. A few of these items are so specific that they can only be purchased from mushroom cultivation supply houses, but most can easily be found at a variety of local sources. Many of the materials you need are also sold for some other, more prosaic purpose; while making shopping easier, this has the additional benefit of providing good cover for those wishing to keep a low profile on their cultivation activities. Hardware stores, kitchen, and restaurant supply houses, pet stores, home brewing suppliers, and garden centers are the treasure troves of the clandestine (or simply frugal) mushroom cultivator.Whenever possible, we have tried to provide multiple general sources for each of the items you might need. Here is a key to the locations where supplies can be found to help you shop.
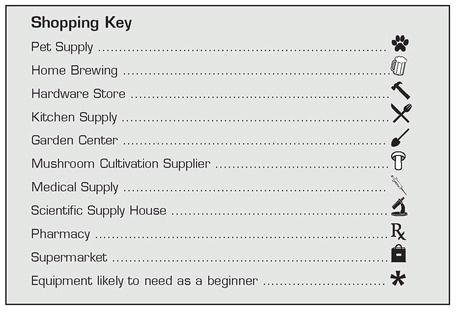
This is a comprehensive list of things you might use along the way.You will only need some of these items before you begin. This is yet another reason we suggest that you first read the book in its entirely, decide what scale you feel comfortable with (hint, hint: start small), plan your experiments, and only then go shopping. There’s no sense in buying 400 pounds of alder sawdust only to have it sitting in your garage for the next ten years. With this in mind, we have placed an asterisk next to those items you need right from the outset.
Equipment
Pressure Cooker

This will be one of the most used items in your cultivation tool shed, so it is important to get a decent one right from the start. Since you will use it to sterilize relatively large items, and in quantity, size is critical. If you can afford a larger unit than you initially need, get it, since you will likely want to upgrade later anyway.The key determinant for what size you should get is the number of quart jars you can safely sterilize at one time. Since mason jars are irregularly shaped, relatively few can fit comfortably inside even the largest pressure cooker, limiting the amount of material you can process at one time. Therefore, we recommend getting a unit that can hold seven or more quart jars at once; the model we use, the All American #941, holds more than twice that many.
As for what brand and type to get, there are many options, but one stands out from the crowd: the All American brand, manufactured by the Wisconsin Aluminum Foundry. All American pressure cookers are the best made, most reliable, and safest available. The company has been in business for many years, and the design of their pressure cookers has not changed significantly since they were first introduced.They are made almost entirely of heavy-gauge cast aluminum, and they have no rubber seals or other parts that can wear out. Replacement parts are readily available, and even a 20-year-old unit bought at a thrift store or on eBay can be made to function as good as new. Unlike smaller, lower-priced kitchen pressure cookers, which lack a way of precisely determining the internal pressure, All Americans have a large, highly accurate dial gauge. They are also designed to hold a vacuum upon cooling, which is essential to avoid the introduction of non-sterile air into your cultures.
There are two general types of pressure cookers to choose from: those that have a steam release valve of some kind, and those that have a metal weighted “rocker” that vents steam whenever the pressure goes over a certain threshold. The latter type is to be avoided, if possible, since this rapid release of pressure can cause liquids inside the cooker to boil over, ruining your media and making a considerable mess. Rocker-style pressure cookers are useable, but to avoid these mishaps, they require more careful monitoring during use. (All American makes both types; the stopcock type they call pressure “sterilizers,” while the weighted rocker ones are considered pressure “canners.”)
Whatever brand and model of pressure cooker you choose, make sure it is in good working order, and that you understand its operation and safety features very well (i.e., read the manual). Make sure all the seals and gaskets are in good shape, and that the lid locks tightly to the base. There should not be any steam escaping around the seals when pressurized. If there is, turn off the heat source, allow the cooker to cool down completely, and reseat the lid properly. Running a bead of Vaseline around the inside rim of the metal-on-metal type cookers will ensure a tight fit and help to keep the lid from seizing to the base during use.
Make sure you add a sufficient amount of water to the bottom of the cooker before each use, at least enough to bring the depth to ½ inch. Never place items directly on the bottom of the pressure cooker or let them touch the outer walls, where the temperatures are highest. Most pressure cookers come with a rack or trivet designed to hold their contents over the surface of the water, and the larger All American models have basket-shaped liners to keep items from direct contact with the cooker itself.
Let the pressure cooker come up to temperature slowly in the beginning. Overly rapid or uneven heating can cause containers to crack or burst.
Always bring the cooker to a full head of steam before closing the stopcock to displace pockets of cooler air. This can take awhile, especially on larger cookers.You should see an unbroken stream of steam escaping from the stopcock vent before closing the valve.
Never leave a pressurized cooker unattended. The temperature and pressure inside a cooker can fluctuate erratically, particularly during the early stages of heating, before the cooker has yet to fully equalize. In order to prevent an explosion and insure complete sterilization, it is essential that the cooker remain at the desired pressure for the full cycle. Check it every ten minutes or so to make sure it isn’t under- or overheating, and adjust the heat source as needed.
Always allow the cooker to cool gradually and on its own. Never touch the outside of the cooker when it is pressurized, and don’t use cold water to cool it more quickly. This can cause the cooker to implode and violently release its contents. At the very least it will produce a large amount of dangerous steam.
To prevent unsterile air from being sucked into the pressure cooker when opened, wrap an alcohol-soaked paper towel around the valve before venting it to release any remaining pressure.
Pressure cookers are potentially dangerous things. They produce high temperatures and steam capable of causing injury. Like a well-honed knife, a pressure cooker is a tool that demands respect and caution, and provides great benefit in return.
Petri Dishes


Petri dishes are shallow, see-through plastic or glass dishes with a loose-fitting cover. They come in a variety of sizes, but the most useful size for fungal cultures is 100 x 15 mm. Reusable glass or Pyrex dishes are long lasting and autoclavable, but are relatively expensive. Pre-sterilized disposable polystyrene dishes come in sleeves of 20 or 25. They are economical, but since they are designed to be used only once and then discarded, they aren’t exactly environmentally friendly.
Both types of Petri dishes can be resterilized using hydrogen peroxide and a microwave oven:
1. Wash the plates thoroughly with dishwashing detergent, taking special care to completely remove any remaining agar.
2. Pour a small amount of 3% peroxide into each dish and swirl it around to expose it to the entire inside surface of the plate. Repeat with its cover, and place it on the dish.
3. Place the stack of dishes in the microwave and heat on medium power until all of the peroxide on the plates has been driven off.
4. Use the plates immediately, or store them in a clean plastic bag until needed.
This procedure is most effective when combined with the use of peroxide in agar cultures.When working with agar lacking peroxide, it is safer to use pre-sterilized plastic or autoclaved glass dishes.
Whenever possible, we recommend the addition of hydrogen peroxide to your cultures to minimize contamination and allow you to work with agar in a less-than-pristine environment. However, peroxide cannot be used in certain situations, such as when germinating spores. In these cases, we have found that 50 mm diameter plates are easier to keep sterile, owing to their reduced surface area.
If Petri dishes are unavailable, you can use 4-ounce jelly jars or similar heatproof glass containers. These have the advantage of being reusable, but they lack see-through lids and take up more than twice as much space as culture plates.
Media Flasks

Media flasks are used for holding liquid media during sterilization and pouring Petri plates. Any thick-walled, autoclavable glass bottle will do, though try to find one with a relatively narrow neck to facilitate pouring. One and a half-liter apple juice or sparkling water bottles with screw top caps are ideal for this purpose.
Mason Jars

We use standard Ball-style mason canning jars, mostly in quart sizes.They are readily available, sturdy, and can be reused indefinitely. For grain spawn, narrow-mouth (70 mm) quart jars are ideal. If you want to try the so-called “PF” technique (chapter 6), you’ll need straight-sided, half-pint jelly jars.
A note of caution: mason jars are durable, but they do sometimes break. Always inspect them closely before use for cracks, discard suspicious looking ones immediately, and be extra careful when shaking jars of grain. Don’t slap them down onto the palm of your hand to separate the grains; a cracked jar can take a finger right off. Instead, simply hold the jar by the lid end and shake it up and down. If the grain is really tightly bound, and you must break it up forcibly, carefully bang the jar against a clean towel supported by a thick pillow or against a partially used roll of duct tape until it loosens up. (Make sure that the lid is tightly sealed as well; you don’t want your precious spawn flying everywhere.)
Always allow your pressure cooker to heat up gradually; rapid heating can cause jars to crack due to the temperature difference between their insides and outsides.
Mason Jar Lids

Don’t bother with the two-piece supplied metal lids; save those for canning tomatoes. For culture work we use a one-piece plastic lid that is heat-resistant and easily modified to allow proper gas exchange. Ball makes a plastic “Storage Cap.” Even though the packaging states that these caps are “not for processing,” they are in fact autoclavable.
To modify these lids, you should carefully drill or cut a 1-inch hole in the center of each cap.When fitted with a filter disc (see next entry), these modified caps allow gases (but not contaminants) to pass in and out of the jar, so your cultures can breathe freely.
Filter Discs

Placed between the lid and the mouth of the jar, filter discs allow gas exchange without the introduction of contaminants. They are made of a synthetic fiber that is heat resistant and can be sterilized repeatedly. They are several millimeters thick and come pre-cut to fit the appropriate jar-and-lid combination.
They sometimes discolor when in contact with substrate or mold spores. In that case, simply soak them overnight in a ¼-strength bleach solution (i.e., ¼-cup regular-strength bleach in ¾-cup water.)
An economical alternative to these discs is Tyvek, which can be cut to fit over the lid of the jar.Tyvek is a synthetic material that is used in a variety of applications. Rolls can be purchased from building supply stores, and smaller amounts are available for free from FedEx or the U.S. Post Office in the form of those oversized, indestructible mailing envelopes.
Because Tyvek is thinner and more flexible than the commercial filter discs, it should be cut in circles wider than the jar mouth, and should hang off the edge of the jar by at least one inch. Tyvek is reusable too, but it should be discarded after three or four uses.

Spawn Bags

Also known as filter patch bags, these are clear, heat-resistant, gusseted flexible plastic bags used for holding large quantities of spawn. They are autoclavable, and have a small square filter on one side for gas exchange. They are loaded with substrate, sterilized, inoculated, and then sealed with an impulse (heat) sealer.
They are ideal for growing out large quantities of spawn because they are flexible and the internal contents can be easily manipulated or examined for contaminants.They lose their elasticity upon heating, and are generally good for only a single use, but bags in good shape can be cleaned out thoroughly and sterilized once more.
We have seen growers use grocery store “oven bags” for spawn. While these are autoclavable and can be made to work, they lack a filter patch, providing less than ideal gas exchange, and they are too thin to heat seal. One way to provide this type of bag with some breathability is to wrap its neck tightly around a thick wad of polyfill or cotton and seal it tightly with a heavy rubber band.You cannot easily shake oven bags to redistribute the contents, since it could pull off the filter plug. Instead, it is best to gently manipulate the spawn with your hands from the outside of the bag.
If you don’t want to use bags for larger amounts of spawn, large mason jars in half-gallon or one-gallon sizes are also useable.
Impulse Sealer


Impulse sealers are used for sealing spawn bags. Be sure to get one wide enough to straddle the entire bag when stretched flat, at least 12 inches across. EBay is a good place to look for deals on impulse sealers.
Alcohol Lamp

This is a glass lamp with a cotton wick and metal collar. Filled with rubbing alcohol, it provides a clean flame for sterilizing scalpels and inoculation loops as you work.
Alternatively, you can use a:
Minitorch

Sold in kitchen supply houses for caramelizing the crust on crème brulée, and sold in electronics stores for soldering, these mini butane torches are useful for sterilizing tools as you work. A good quality one will have a solid base to keep it upright while it sits on the bench top.
Balance

Mechanical or electronic models are equally good. The important qualities to look for in a balance are accuracy to at least 0.5 g, the ability to weigh up to a minimum of 250 g (1 kg is better) and a pan large enough to accommodate oversized items.
Scalpel

Scalpels are used for cutting and transferring agar and tissue cultures. A thin-handled dissecting scalpel with disposable #10-sized blades is ideal. If you can’t find these, an all-aluminum X- acto-style knife will work okay, though it can be somewhat harder to maneuver into tight spaces.
Inoculation Loop

This wire loop at the end of a metal or wooden handle is used to transfer spores or small amounts of mycelium to agar plates. It can be found in scientific or brewmaking supply stores, or made from a dowel and a piece of thin stiff wire. An inoculation loop is not needed if you utilize the “cardboard disc” method of spore germination.
Sharpies
These permanent, write-anywhere markers are essential for labeling culture containers of all kinds.
Funnels

It is helpful to have two types of plastic or metal funnels: a narrow-necked one for pouring liquids and fine powders, and a wide-mouthed one for filling jars.
Measuring (Serological) Pipette & Rubber Bulb
If you plan to work with agar, you’ll need some way of measuring small volumes of liquid (1-15 mL) to add to your cultures. Ten-milliliter glass measuring pipettes are ideal for this purpose since they are autoclavable, reusable, and have markings on them to easily determine volume. A rubber bulb is used to draw and dispense liquids from the pipette. Both can be found at scientific suppliers and some homebrew stores.
A glass 10-milliliter graduated cylinder or a set of metal measuring spoons can be used for this purpose, but will require more handling and care to avoid contaminating your cultures while you work.
Graduated Cylinders


These are used to accurately measure liquids. Cylinders in 1-liter, 100-milliliter, and 10-milliliter sizes should cover all the bases.
Measuring Cups & Spoons

Measuring cups and spoons can be used instead of graduated cylinders, but are somewhat less accurate. Use Pyrex ones for 1-cup (250ml) to 8-cup (2 L) amounts, and metal ones for smaller volumes. Both types can be autoclaved or sterilized in boiling water (5 minutes at a rolling boil) when sterility is required before use.
Syringes

Syringes are used for doing spore mass inoculation, à la the “Psilocybe Fanaticus” technique. Ten- or twenty-milliliter sizes are used, with wide bore (18-gauge) needles. They can be repeatedly autoclaved in a pressure cooker or sterilized in boiling water as above.
Syringes can be purchased from surgical and veterinary supply stores, and from some online mushroom supply vendors. However, their sale is regulated in many U.S. states, and they can sometimes be hard to find locally.
If you buy pre-filled spore syringes, clean and save the syringe and needle after use. They are autoclavable and can be reused many times.
Supplies
Hydrogen Peroxide (3%)
This antiseptic is added to cultures to protect them from contamination. It is available at most pharmacies or grocery stores. The actual concentration of hydrogen peroxide solutions sometimes varies, so make sure the date on the bottle is of recent vintage (See sidebar below for a method of finding hydrogen peroxide’s concentration level). In this concentration, peroxide is relatively innocuous to human health and requires no special handling methods, aside from wearing gloves. It is a mild bleaching agent, so be careful not to drip it onto clothing.
More concentrated (8-35%) solutions are available from a variety of sources, such as pool supply stores, and online. Hydrogen peroxide in concentrations greater than 3% can cause severe burns and is potentially flammable, so be very careful when working with it.
Peroxide degrades fairly rapidly, so in order to insure that it remains at the proper concentration, use the bottle as soon as possible after opening. Between uses, wrap the neck and cap in parafilm or plastic wrap, and store the bottle inside a clean plastic bag in the refrigerator.
Before each use, wipe down the outside of the bottle and cap (including the mouth and neck beneath the cap) with alcohol, and take special care not to touch any part of the bottle itself with your hands or tools when dispensing.
Always sterilize pipettes and graduated cylinders that will come into contact with peroxide before use, either in a pressure cooker along with your media or by submerging them in boiling water for 5 minutes.
Determining Peroxide ConcentrationHere is an easy way to determine the precise concentration of hydrogen peroxide in a given solution, a method that is particularly useful when using a brand of unknown quality or vintage.MaterialsA test tubeA small balloonA small slice of a mushroom, or any skinned soft fruit or vegetable, such as a bananaA 100 ml graduated cylinderA large pot or tub of water1. Pipette 5 mL of peroxide into the test tube.2. Holding the tube at a slight angle, place the slice of mushroom in the neck of the tube, without letting it fall into the peroxide.3. Flatten the balloon to remove any air left in it, slip it over the neck of the tube, and then let the mushroom slice fall into the peroxide.4. Hold the balloon tightly over the neck of the tube to prevent oxygen from escaping, or tie it on tightly with a piece of string or rubber band.5. Meanwhile, completely submerge a 100 mL graduated cylinder in a large pot of water, making sure to displace any air in the cylinder.6. Agitate the tube occasionally to encourage the decomposition of the peroxide.7. Within five to ten minutes, oxygen evolution should have slowed considerably and the peroxide should be fully decomposed.8. Twist the neck of the balloon closed, and slip it off the test tube.9. Hold the balloon under the water and release all of the oxygen into the mouth of the inverted cylinder.10. While keeping the open mouth of the cylinder fully submerged, invert it completely, and read the amount of oxygen it contains.Balloon filling with oxygen.Reading the amount of oxygen in the graduated cylinder.Five mL of 3% hydrogen peroxide should theoretically release 49mL of oxygen when fully decomposed. As long as yours comes to within 5 mL of theoretical, it should be fine. If not, then divide the amount you actually got by 49mL, and multiply that number by 3% (0.03) to determine the actual percentage of peroxide present. To determine the amount of peroxide to use in a given recipe, divide the actual concentration of peroxide (determined in the previous equation) by 3% and multiply that number by the amount of 3% required.Here’s an example: say your peroxide released 33ml of oxygen, and the recipe calls for 10 mL of 3% hydrogen peroxide:33mL ÷ 49mL = 0.673 * 3% = 2.02% H2O2 3.00% ÷ 2.02% = 1.48 * 10mL = 11.8mL
Isopropyl (Rubbing) Alcohol
This is used for disinfecting hands, surfaces and containers, and as fuel for alcohol lamps. It is available in grocery stores and pharmacies in either 70% or 91% concentrations, either of which is suitable.
Warning: Isopropanol is highly flammable!! Keep it away from open flames, please. Make sure whatever alcohol you have used has fully evaporated before you light your alcohol lamp.
Bleach
Regular-strength laundry bleach is useful for cleaning surfaces and tools. Avoid brands with added detergents. Dilute to at least ¼-strength before use. A 10% strength solution in a spray bottle is an excellent surface and air disinfectant.
Parafilm

Parafilm is a paraffin-based, elastic film used to seal Petri dishes. It is gas permeable, which means that it allows for gas exchange while keeping contaminants out of cultures. A convenient form in 1-inch-wide rolls is sold by some garden supply vendors as “Grafting Tape.”
If you cannot find Parafilm, you can substitute polyethylene cling film, such as Glad Wrap (but not Saran Wrap or similar brands, which are made from polyvinylchloride and are not gas permeable.). Using a very sharp knife, carefully cut a 1- to 2-inch-wide section off the end of a full roll.
Surgical Gloves
Disposable latex gloves are essential for keeping grubby hands away from your pristine cultures. They need not be pre-sterilized. Just wash your hands and arms well before putting them on, then wipe the outside of the gloves with an alcohol-soaked paper towel (always allow them to dry completely before going anywhere near an open flame.)
Substrates & Casing Materials
Whole Grains

For spawn production, the most commonly used substrate is whole grain. Whole grains make an ideal medium for spawn for a number of reasons. Each grain acts like a miniature capsule of nutrients, minerals, and water that is easily colonized by higher fungi, while its fibrous husk partially protects it from contamination by other organisms. Upon colonization, the grains are easily separated from one another. Finally, when colonized grain spawn is used to inoculate bulk substrates, each grain serves as a compact container of mycelium and nutrient reserves, a remote outpost from which the fungus can leap off onto the new medium.
While almost any cereal grain will work as spawn, we recommend soft winter (white) wheat, since it has worked well for us, and seems to be free of the bacterial contaminants that can be present on rye and other grains. You may use whatever grains are readily available to you, though we do suggest using larger kernel grains like rye, wheat, or corn, rather than small-grained cereals like millet or rice, which have a tendency to clump together when cooked.
We have seen some growers use wild birdseed mix with good results, which has the obvious advantage of being cheap and readily available. However, because it is a mixture of different-sized grains, birdseed is more difficult to moisten properly. It can also be quite sticky when moist. To minimize these issues, hydrate birdseed with a 24-hour cold soak instead of hot water, and rinse and drain it very well before loading into containers.
You should try to use organic grains whenever possible, since that is the only way to ensure that they have not been treated with fungicides.

Malt Extract, Dried
This is a powdered extract of grains that have been “malted,” or sprouted, to promote partial conversion of their starch into sugars. Malt extract is used in agar media as a primary nutrient source. It is readily available from brewing suppliers. Be sure to use light or tan malt. Darker malts have been caramelized, and fungi don’t grow well on sugars that have been caramelized.
Yeast Extract

A dried extract of yeast cells, rich in vitamins, minerals, and protein, yeast extract is added to agar media as a nutritional supplement. Brewer’s yeast, available in many health food stores, is an acceptable substitute, though it is not as effective as true yeast extract.
Calcium Carbonate (CaCO3)
Calcium carbonate is also known as lime, hydrated lime, limestone flour, oyster shell flour, and chalk. It is used to buffer the pH of casing soils and substrates, discourage contamination, and provide calcium to the growing fungus. Fungi tend to prefer slightly basic (i.e. pH > 8) media, while bacteria and some other contaminants do not. Check the label to be sure the calcium carbonate you buy is low in magnesium (< 1%), because some fungi do not grow well on substrates containing high amounts of it.
Calcium Sulfate

Otherwise known as gypsum, calcium sulfate is used to capture excess water in substrates, making them easier to shake or separate, and helping to prevent water logging and contamination. It is essentially neutral in pH, and has no buffering capability.
Hardwood Sawdust and Chips

These are substrates for Psilocybe cyanescens, P. azurescens, and similar lignicolous (wood-inhabiting) species. Although most hardwood species will do, alder, cottonwood, oak, birch, and beech are ideal. If you have any of these tree species growing nearby, you might be able to get fresh chips from your local highway department or garden center, or you could chip your own. Chips produced from trees in winter or early spring are best, since they will be highest in sugars and contain a minimum of leafy matter, which can be a vector for contamination in beds.
Sometimes you can get hardwood chips locally through barbeque suppliers, who sell them for use in food smokers If you do not have access to hardwoods locally, wood chips can be purchased online. Finely chipped wood chips of beech or maple are sold as animal bedding (Beta-Chip and SaniChip are two brands to look out for), but these are generally too fine to use alone, and must be combined with larger chips of some kind.
Sawdust Fuel Pellets
Used in special wood stoves for home heating, these are made from sawdust that has been compressed into small pellets. The high heat generated during manufacturing renders them more or less sterile.When moistened with warm water, the pellets expand into sawdust again. This product is available at home heating suppliers and some hardware stores. Stove pellets make a good source of sawdust for substrates—just be sure to get a brand made exclusively from hardwoods.They are also sold as fuel for food smokers in a variety of tree species, including alder and oak.

Oak stove pellets, birch dowels, and alder wood chips
Spiral-Grooved Dowels

Spiral-grooved dowels are readily available from woodworking suppliers as furniture-joining pegs; the best ones for mushroom use are 1 to 2 inches long and ¼-inch or  -inch in diameter. They are usually made from birch, and will be designated as such. They are most commonly used in mushroom cultivation on logs, where the colonized pegs are pounded into holes around the circumference of the log. The spiral groove around the outside of the peg provides a maximal surface area from which the mycelium can leap off onto subsequent substrates.
-inch in diameter. They are usually made from birch, and will be designated as such. They are most commonly used in mushroom cultivation on logs, where the colonized pegs are pounded into holes around the circumference of the log. The spiral groove around the outside of the peg provides a maximal surface area from which the mycelium can leap off onto subsequent substrates.
 -inch in diameter. They are usually made from birch, and will be designated as such. They are most commonly used in mushroom cultivation on logs, where the colonized pegs are pounded into holes around the circumference of the log. The spiral groove around the outside of the peg provides a maximal surface area from which the mycelium can leap off onto subsequent substrates.
-inch in diameter. They are usually made from birch, and will be designated as such. They are most commonly used in mushroom cultivation on logs, where the colonized pegs are pounded into holes around the circumference of the log. The spiral groove around the outside of the peg provides a maximal surface area from which the mycelium can leap off onto subsequent substrates.
Paper Pellet Cat Litter

Used for the storage of cultures in small glass tubes. The loose, open structure and limited nutritional content of paper makes it ideal for the long-term maintenance of most mushroom species. Look for a brand that is unscented and made of 100% recycled paper; Crown, Good Mews, and Yesterday’s News are three brands we have found to be effective.
Peat Moss
A component of casing soil (see chapter 10), peat moss is sold in garden centers everywhere. While it has little nutritional value, its high water-holding capacity provides moisture to the developing fruitbodies. It is somewhat acidic and must be buffered with calcium carbonate.
Vermiculite

Vermiculite is another component of casing soil. It is used for its water-holding capacity, and its fluffy, open structure, which allows proper gas exchange. It is available at many garden suppliers. Use the coarsest grade you can find.

peat moss and vermiculite
There is a myth that vermiculite contains asbestos, which is not true. Apparently, there was a single vermiculite mine in Montana that was contaminated with asbestos, and it was shut down. After this incident was publicized, other manufacturers began testing their vermiculite for asbestos, but it was not found anywhere else. Nevertheless, vermiculite and similar materials (like peat and calcium salts) do contain a lot of very fine particulates, which can be harmful if inhaled. Always wear a painter’s dust mask when handling them in their dry state.
Water Crystals
Made from a synthetic polymer chemically related to super glue, these crystals can absorb four hundred times their weight in water, and then release it slowly back into their surroundings. When fully hydrated, they look like clear gelatin.They are used in agriculture and gardening to conserve water usage and to protect plants from drying out completely between waterings. In mushroom cultivation, they are added to casing soil to help maintain adequate moisture levels. The crystals come in two varieties: those made from sodium or others made from potassium. Since high levels of sodium are harmful to many fungi, be sure to get the kind made from potassium. (One potassium-based brand is called “Terra-Sorb”).

“Water crystals” in their dry state.
Spores
Of course, none of any of these materials are of any use to you if you don’t have mushroom spores to grow on them.Why then, you ask, didn’t we tell you first where to get your hands on some Psilocybe spores?
It’s a good question, and one that calls for a complicated answer, unfortunately. More likely than not you live in a country that has (however ridiculous the notion) deemed these humble and gentle mushrooms illegal. While the authorities can do little to truly contain these organisms (no more so than if they were to ban the mold growing on your shower curtain), they could very well imprison you for cavorting with them. That is a sad truth worth contemplating awhile before you choose to do so.
If, in the end, you decide that the rewards outweigh the risks (as we believe they do), we urge you to exercise caution when doing anything illicit. Keep a low profile; be discreet. Don’t talk to others about your new hobby (or at least not unless you know for absolute sure they are “cool”). Come up with plausible alternative meanings for your purchases, even if you only tell them to yourself: the 50-pound bag of wheat berries is to grind your own bread flour, the malt is for your next batch of homebrewed chocolate porter, and so on. Don’t brag about your exploits on the Internet; however anonymous you think you are: it is a fact that anything you do online nowadays can be traced back to your computer, if someone wanted to. It might require a court order, but it’s still better not to take the risk.
Don’t start this hobby with the notion that in no time you’ll become the local psychedelic mushroom kingpin, a fungal Tony Montana. The profit motive has led countless individuals astray, as it could you. Don’t sell the fruits of your labor. If you must share them, give them away to close friends, or better yet, teach them to grow their own. (Give a man a fish . . .)
But still, you ask, where do I get the spores to grow them? Yes, yes, we’re getting there. We said it was a complicated answer, didn’t we?

Spore prints on aluminum foil.
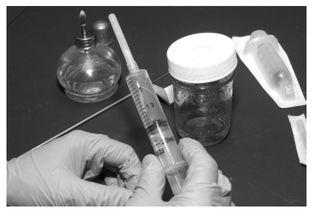
A homemade spore syringe
Ideally, you know someone who grows or has grown these mushrooms, and he or she will give you a spore print or two to get you started, along with some advice (and better yet, a copy of this book). Perhaps you live in a region where these mushrooms grow wild (the Gulf Coast states for Psilocybe cubensis and the Pacific Northwest, for P. azurescens and other wood lovers), and you can collect fresh specimens to clone or print.
If not, then you are, like most people, left with one option: purchase your spores online or by mail order and jeopardize your anonymity. If you must do so, exercise caution. Research your source well; the most reputable vendors are likely to have been in business for some time, and are in the business of being discreet.The best ones accept cash payments in the mail, and claim to destroy all customer records after fulfilling the order. Use them, and only pay cash.
To this end, we have provided in appendix C a list of vendors we consider trustworthy and discreet based upon our own research (at the time of this writing, at least).
Chapter 3Psilocybe: The SpeciesPsilocybe cubensisA flush of P. cubensis.P. cubensis fruitbodies displaying a strong bluing reaction where they were handled.A close-up of the gills of a P. cubensis fruitbody.Psilocybe azurescensPsilocybe cyanescens photo: Peter WernerPsilocybe cyanofibrillosa photo: Peter WernerPsilocybe subaeruginosaChapter 6Improved PF Tek instructions (described on pp. 85-87)1: Materials.2: The vermiculite contains a slight excess of water.3: After the reserved material has been mixed in, the vermiculite is at field capacity.4: The rice flour is added to the vermiculite.5: The completed PF substrate.6: Loading the jars with substrate.7: Adding the dry casing layer. The jar on the left has been cased, while the one on the right has not.8: Cased PF jars.9: Adding the foil covers and plastic lids.10: PF jars loaded into a small pressure cooker.PF Tek Inoculation (described on p. 87)1: Materials.2: Heating the needle in the flame of an alcohol lamp.3: Injecting the jars.4: Inserting the tip of the needle through the casing layer into the substrate below.PF Tek Colonization/Germination (described on p. 88)Spore germination at several points around a PF jar.Two individual colonies of mycelium approaching one another.A fully colonized PF jar.PF Tek: Preparing Jars for Fruiting (described on p. 90)A PF jar ready for fruiting. Note the mycelial fans poking up through the casing layer.PF jars in their fruiting chamber.Mushroom primordia emerging from beneath the casing layer.PF Tek Harvest (described on pp. 90-91)PF jar ready to be harvested.Mushrooms at their ideal stage for harvest, before their partial veils have broken.After the partial veil has broken, its remnants remain attached to the stipe in a skirt-like ring known as an annulus.PF Tek: Making Spore Syringes (described on pp. 92-93)1: Materials.2: Heating the loop in the alcohol lamp.3: Cooling the loop in the sterile water.4: Picking up spores on the inoculation loop.5: Adding spores to the jar.6: Filling and emptying the syringe several times to create an even distribution of spores.7: A completed syringe, ready for use.Chapter 7Making Malt Yeast Agar (MYA) Medium (described on pp. 95-97)1: Materials.2: Warm, sterilized agar ready for addition of peroxide.3: Dispensing peroxide into the warm agar medium.4: Pouring agar plates.5: Cooling the Petri dishes. The glasses of hot water prevent condensation from forming on the upper plates.6: Peroxide plates drying.7: Plates in their sleeve, ready for storage until use.Cardboard Disc Spore Germination (described on p. 100)1: Sterile cardboard discs and tubes of malt-yeast solution ready for inoculation.2: Inoculated cardboard discs in a malt-yeast extract tube. The arrow indicates the cluster of spores on the edge of a disc.3: Spores beginning to germinate on the discs.4: Fully colonized spore discs.5: Colonized spore disc after transfer to a peroxide agar plate.6: Spore disc culture colonizing an agar plate.Tissue Transfers (Cloning) (described on pp. 101-103)1: Materials.2: Sterilizing the outer surfaces of the mushroom with alcohol.3 & 4: Slicing and splitting open the mushroom to expose the sterile mycelium within.5: Cutting a piece of mycelium from the inside of the stipe.6: Transferring the chunk of mycelium to an agar plate.7: Mycelium from a tissue fragment colonizing an agar plate.Agar-to-Agar Transfers (described on pp. 103-104)1: Materials.2: Cooling the sterile scalpel blade in a blank Petri dish.3: Cutting a wedge of mycelium on agar.4: Agar wedges ready for transfer.5: Placing the agar wedge face down onto the new plate.6: The original and new culture plates.Paper Pellet Storage Medium (described on pp. 106-107)1: Paper pellet tubes ready for sterilization.2: Flaming the neck of a test tube in an alcohol lamp.3: An agar wedge of mycelium beginning to colonize the paper substrate.4: A fully colonized paper pellet culture tube.5: A paper pellet culture beginning to colonize a new agar plate.Chapter 8Agar-to-Grain Transfers (described on 111-112)1: Materials.2: Dispensing peroxide into grain jars.3: After shaking, the grain is left on a slope.4: Cutting agar wedges.5: An agar plate ready to be used to inoculate grain jars. The uncolonized border and the original culture at the center of the plate will not be used.6: Adding an agar wedge to a grain jar.7: After inoculation, the hill of grains is gently tipped back over the agar wedges.Shaking Grain Jars (described on p. 113)1: Mycelium beginning to leap off the agar wedges onto the grain.2: A partially colonized grain jar.3: A fully colonized grain jar.Understanding Contamination on Grain (described on p. 113)A grain spawn bag showing signs of contamination with a blue mold.Inoculating Grain Bags Bags (described on p. 117)1: Measuring peroxide in a sterile graduated cylinder.2: Pouring peroxide into the grain bag.3: Pouring colonized grain into a grain bag.4: Sealing the bag with a heat sealer.Chapter 9Fruiting Containers (described on p. 118)A variety of suitable fruiting containers.Chapter 11Fruiting and Harvesting (described on p. 129)P. cubensis primordia emerging from the casing layer.P. cubensis fruiting from a 50/50 casing layer.Chapter 13Outdoor CultivationP. azurescens fruiting from wood chips beneath a layer of grass.Mycelium and AgarMycelium growing onto cardboard from a stem butt of Psilocybe azurescens (pp. 138, 152-153).An agar culture of P. azurescens (p. 139).P. azurescens and P. cubensis Rhizomorphs (described on p. 140)1: P. azurescens rhizomorphs on cardboard.2: P. cubensis rhizomorphs on grain.3: A network of rhizomorphs hangs from the base of a freshly picked mushroom.4: Rhizomorphs of P. azurescens on unsterilized wood chips, forever in search of their next meal.Wood-Based Primary Spawn (described on p. 141-143, 145-146)1: Bags and jars of wood chips and dowels ready for sterilization.2,3: Mycelium leaping off onto dowels and wood chips.4: A bag of colonized birch dowels ready for further use.Outdoor Contaminants (described on p. 146)1: Trichoderma viride growing on wood.2: Wood chips and sawdust contaminated by brown-rot fungi.3: Trichoderma viride contamination on a casing layer.P. azurescens from Spawn to Bed (Secondary Spawn, described on pp. 147-148)1: The clean tub is lined with a single sheet of cardboard.2: A portion of the spawn is laid over the substrate mixture.3: The completed substrate.4: Substrate beginning to be colonized. Note how the mycelium has also started growing on the cardboard covering.5: Close up of mycelium beginning to colonize secondary substrate.Casing & Companion Planting (p. 150)Below: P. azurescens fruiting from wood chips buried beneath grass.Chapter 16Conclusion (These edible species are described on p.167.)Above: Wine-cap stropharia (Stropharia rugosoannulata) fruiting from a bed of alder chips cased with peat moss.Right: Cinnamon caps (Hypholoma sublateritum) fruiting from a bed of alder chips.
6
PF TEK IMPROVED
In this chapter, we describe the popular “Psilocybe Fanaticus” technique of mushroom cultivation, otherwise known as the “PF Tek,” along with our refinements of it. For many Psilocybe mushroom cultivators nowadays, this method is the first system they are exposed to, so we felt it was important that we cover it in our book, even if we do not recommend it as a general practice. Since it is as close to foolproof as a mushroom cultivation method can get, it is a good place for a beginner to start. It requires a minimum investment of time and expense and exposes the novice to the entire life cycle of a mushroom in a relatively short time. Seeing this process up close and firsthand will allow you to understand the principles underlying the more complex procedures that follow. If you choose to begin your mushroom cultivation with a Psilocybe cubensis spore-water syringe rather than a spore print, this method is a good way to put it to use (though not the only one: you could also use it to directly inoculate grain spawn, as we explain in chapter 8). The technique allows one to produce a pure fruiting strain of P. cubensis very quickly, with a minimum of intervening steps. You can take the best specimens from your jars and clone them onto agar. As soon as you have isolated a pure strain, you can leave the PF Tek behind, in favor of more efficient and versatile methods.
Even though this method is quite simple and virtually foolproof, it does have a number of drawbacks. First of all, the mushrooms are grown out on a mixture of brown rice flour and vermiculite in ½-pint containers. By weight and volume, these substrates contain relatively little in the way of nutrition, and therefore yields are correspondingly low. Similar volumes of wheat berries can support far larger fruitings, and can be processed in much greater quantities. Second, the method relies upon the use of spore-water syringes.While you can make your own (see p. 92) if you have a source for both spores and syringes, most who use this method buy them pre-made from spore suppliers. As we have already mentioned, we think it best to patronize these companies as little as possible. Learning traditional agar and grain methods allows you to work with materials that can be purchased from less risky sources. Lastly, the “PF Tek” is not adaptable to the other species of Psilocybe we describe in this book. It relies on the unique fact that P. cubensis will fruit on a wide variety of substrates and under many different environmental conditions. Other species are much more particular in their demands and won’t yield their secrets so readily. (You can use the PF Tek to grow the mycelium of other species, but not to produce fruits.) If you’d like to grow a bed of P. azurescens in your back yard, or almost any edible mushroom, indoors or out, you will have to familiarize yourself with the more universal methods we describe later in the book.
These caveats aside, here’s the basic idea behind the original PF Technique: a mixture of brown rice flour and vermiculite is moistened, then loaded into ½-pint jars. A thin layer of dry vermiculite is placed over this, followed by a few layers of aluminum foil. The brown rice flour provides a balanced nutrient base for the fungus to colonize, while the vermiculite serves as a reserve for water and helps to create an open, airy structure, allowing the growing cultures to breathe. The jars are then sterilized in a pressure cooker or a boiling water bath. After cooling, the first layer of foil is removed, and the jars are quickly injected with a few milliliters of a spore solution from a syringe at several points around the circumference of the jar. The top foil layer is replaced, and then the jars are placed in an incubator or a warm, draft-free spot.
In time, the spores will germinate and fuse with a suitable mate to form a dikaryotic mycelium.The large numbers of spores in each injection insures that mating will occur, and that each jar contains a wide variety of strains.The many strains present compete to colonize the jar, with the most vigorous (and by extension, most likely to fruit well) overtaking the weaker ones. After several weeks or so, the jars should be fully colonized, and are ready to be fruited.
At this point in the original PF Tek, the “cakes” of mycelium are knocked out of the jars and placed upside down on a bed of moistened perlite at the bottom of a clear container such as an aquarium or a plastic storage bin, which is covered to maintain high humidity levels. The fruiting chamber is placed beneath a light source (fluorescent grow lights connected to a timer or even a brightly lit window). The cover is removed once or twice a day, and the cakes are fanned by hand to remove built-up CO2, then misted with water from a hand spray bottle. In time, primordia form on the outer surface of the cake and eventually mature into full-sized mushrooms.
In our “improved” PF Tek, we leave the substrate in the jar, and mushrooms fruit only from the top surface of the jar. This serves a number of purposes. One, it eliminates the need for elaborate and messy tubs of perlite. Instead, the jars are placed into any clear enclosed container, or even a plastic bag, perforated to allow gas exchange. Two, the need for high ambient humidity is reduced, because the top layer of pure vermiculite acts as a casing layer, holding a reservoir of water that the developing fruits can draw upon. Since fruiting is restricted to a horizontal surface, the mushrooms that form retain a much more natural appearance and shape. The original “cake” method, on the other hand, tends to produce fruits of bizarre shapes and sizes, since they form at random points around the cake. (Since spores are most efficiently dispersed from downward facing gills, most mushrooms use gravity to orient themselves horizontally. If, as in our method, the stipes are already pointing in the right direction, they naturally grow straight and tall.) With the incorporation of a casing layer, the “improved” PF Tek more closely resembles the advanced methods we present later. After you have performed this method once or twice, you will be more than familiar with the basic mushroom life cycle, and ready to move on.
The “Improved” PF Tek
For photos of this process, see pp. 58-59.
Materials
40mL (scant ¼ cup) organic brown rice flour (per jar)
140ml (½ cup) vermiculite (per jar), plus extra for casing layer
Water
½-pint (~250mL) mason jars
Aluminum foil
Spore-water syringe(s)
Alcohol lamp or butane lighter
Rubbing alcohol
140ml (½ cup) vermiculite (per jar), plus extra for casing layer
Water
½-pint (~250mL) mason jars
Aluminum foil
Spore-water syringe(s)
Alcohol lamp or butane lighter
Rubbing alcohol
Material Notes: Brown rice flour is available at some health food stores, or you can grind your own in a mini coffee grinder or spice mill. The jars should be straight sided (jelly jars), without the shoulders present on larger-sized canning jars. Tap water is fine, but you can use bottled or distilled if your water source is suspect.
Sterilization Note: This method contains the one instance in this book where we describe a boiling water bath sterilization of a substrate as an alternative to pressure-cooking.The water bath process is effective, but not 100% reliable; some percentage of jars prepared with it will still be likely to contaminate. If you have a pressure cooker, you should use it here too; if not, now is as good a time as any to get one.
1.Depending on the number of jars you will inoculate (a 10 mL syringe contains enough solution to inoculate 8-10 jars), place the required amount of vermiculite in a bowl. Now remove approximately 5% of this and place it in a separate container.
2.Into the main bowl of vermiculite, add water a little at a time, stirring as you go, until the mixture can hold no more and there is just a slight excess of water at the bottom of the bowl. Now add the reserved dry material and mix thoroughly. The vermiculite should now be at “field capacity,” meaning that it contains the maximum amount of water it can comfortably hold.
3.Pour the brown rice flour into the bowl and mix well, coating the vermiculite grains with a layer of moistened flour.
4.Spoon this mixture into the jars, leaving a level ½-inch (1-centimeter) gap at the top. Place it into the jars loosely and do not pack it down; keeping an open, airy structure will allow the mycelium to breath and grow at an optimum rate.Take a damp paper towel and wipe down the sides and around the inside rims of the jars to thoroughly remove any stray substrate, which could otherwise become a source of contamination.
5.Fill the remaining space of the jar with dry vermiculite.This layer will initially act as a barrier to contaminants, which, should they somehow find their way inside the jar, would be prevented from coming into contact with the substrate. Later, it will serve as the casing soil, from which the mushrooms will fruit.
6.Take two 5-inch square pieces of aluminum foil and wrap them tightly over the mouth of the jar. Loosely screw lids onto the jars, taking care not to tear the foil below. (The use of lids in the PF Tek is optional, but does provide an additional layer of protection.)
7.Load the jars into your pressure cooker, along with the appropriate amount of water, and sterilize the jars at 15 psi for 45 minutes. If there is enough room, the jars may be stacked in more than one layer. OR (Boiling Water Bath Method):
7.Place the jars in a large cooking pot in a single layer, along with enough water to bring it to about halfway up the sides of the jars. Cover the pot and boil for 1.5 hours. Check occasionally to make sure the water level remains constant, adding water as necessary.
Phase 2: PF Tek Inoculation
For photos of this process, see p. 60.
1. When the jars have cooled to room temperature, place them onto a clean work surface, along with the spore-water syringe and alcohol lamp. Remove the lids, and loosen the top layer of foil.
2. Remove the cover from the syringe, wipe the needle with an alcohol-moistened, clean paper towel or cotton ball, and then hold the tip of the needle in the flame of your lamp until it just begins to glow red (be careful to keep the plastic end of the needle away from the flame, and be sure to exercise caution when using alcohol near an open flame.) Allow it cool for a few seconds before using.
3. Working one jar at a time, remove the top layer of foil, shake the syringe gently to disperse the spore solution, and inject a small amount into the jar at four equally spaced points just inside the inner rim. Insert the needle 1 inch (2 cm) into the jar so that its point is past the dry vermiculite layer, and then squeeze out a few drops.You should be able to see the solution run down the sides of the jar. Repeat at the other three points. Each jar should get a total of 1-1.5 mL of solution.
4. Inject all of the jars in the same way, and then replace the top layer of foil and lid (if using) on each of them. Mark the outside of the jars with relevant information and/or notebook number, and place in a clean, warm spot to incubate.
Phase 3: Incubation
The jars should be incubated in a warm, draft-free location, in the 75-85˚ F range. If the temperature in your home is consistently within this range, then simply storing them in a clean box should be sufficient. If not, an incubator box will insure healthy and rapid growth, and is simple to construct from a cooler and a few items you can purchase from the reptile department of a pet store.
Materials
25-50 gallon plastic or Styrofoam cooler
8-watt reptile heating mat
Adjustable thermostat controller
Air temperature thermometer
8-watt reptile heating mat
Adjustable thermostat controller
Air temperature thermometer

The incubator in use.
Place the heating mat and thermometer in the cooler, plug the mat into the temperature controller, and turn it on. Set the controller on its lowest setting, and let the cooler warm up for a few hours before adjusting it gradually upwards until it reaches a steady 80˚ or so. Situate the heating mat on one side of the cooler, and stack your jars on the opposite side, as far from direct heat as possible.
Depending on the ambient temperature of the room, you may need to occasionally adjust the thermostat to keep a constant temperature inside the cooler. When ambient temperatures go above 85˚, you’ll need to figure out a way to keep the jars from overheating. In this case, rather than constructing an elaborate refrigeration device, your best bet is to store them in a closed container in a cool spot in your home, such as an unheated basement. If no such place is available to you, this would be a good time to take a break for a while, until outside temperatures have cooled sufficiently to resume cultivation.
Phase 4: Germination/Colonization
Within a week or so, you should begin to see the first signs of spore germination in your jars. Look for tiny pinpoints of bright white fuzzy growth, usually near the base of the jar directly below the injection points. In time, these tiny colonies will radiate outward to form individual spheres of mycelium. In 10 days to a few weeks, the spheres inside each jar will join one another, and the jar will be fully colonized. (See color section, p. 61.)
Contamination
If you see any growth in your jars that is not pure white in color, the jar is likely contaminated, and should be removed immediately from the incubator and disposed of. The most common offenders will be molds, which tend to have highly colored spores in hues of blue, green, black, or pink. Bacterial contamination, on the other hand, will appear as spots of wet, sticky blobs on the inner surface of the jar, and may be accompanied by a sour or rotten apple smell.
Always remember to dispose of contaminated culture containers away from your work and growing areas, and clean the containers thoroughly before bringing them back into your workspace.
Phase 5: Fruiting
Once the jars are completely colonized, they are ready to be fruited. At this point, you will remove the lids and foil, moisten the top layer of vermiculite, and place the jars below a light source, either artificial light provided by a dedicated fluorescent “grow” light or a brightly lit window.
Because P. cubensis requires light to stimulate fruiting, and you want to limit fruiting to the upper surface of the cakes, you need to somehow restrict light exposure to only that area of the jars. You can do this in a number of ways, such as wrapping the jars in aluminum foil or strips of thick, opaque paper. One simple method we have used is to place the jars inside short pieces of cardboard tubing, like the type used to store posters, cut to come just above the rim.

A PF jar in a cardboard tube.
Preparing the Jars for Fruiting
For photos of this process, see p. 61.
1. Remove the lids and foil from each jar.You may see some “fans” of mycelium poking up through the vermiculite layer.
2. Wipe a clean fork with some alcohol, allow it to evaporate, and then gently scrape (not scrape off) the dry vermiculite layer all the way down to the top of the cake below, to break up and evenly distribute the mycelial fragments within.
3. Take a spray mister of clean water and mist the vermiculite until it is saturated (it will darken slightly in color; as soon as you can see free flowing water, that is enough.)
4. Repeat with each jar, cleaning the fork each time to avoid inadvertently spreading contaminants.
5. When all of the jars are ready, place them in individual cardboard tubes inside an enclosed container, such as a large clear plastic bag (cut or punch several holes in it to provide some gas exchange) or a clear plastic storage tub.
6. If you are using a fluorescent grow light, set your timer to an 8-hour on /16-hour off cycle; otherwise, simply locate the jars in a well-lit area, such as near a sunny window. The ideal temperature of your growing area should be in the 65-75˚F range, slightly lower than that required during colonization.
7. Mist the casing lightly once or twice each day to replace any water lost to evaporation.
In a few days to two weeks you should see primordia begin to form. Most likely, they will form inside the casing layer and will not be visible until they are already well-formed miniature mushrooms. Once they have achieved this size, they tend to grow astoundingly fast, and can seem to reach full size almost overnight. As they grow, they will draw water from the cake and the casing layer, so be sure to increase misting as needed to keep the vermiculite saturated, always taking care not to over water.
Phase 6: Harvesting
Once the mushroom has reached an appropriate size for efficient spore dispersal, it ceases growing and its cap widens to expose its spore-producing gills to the atmosphere. The best time to harvest your mushrooms is just prior to this point, since a single mushroom can shed an astronomical number of spores, which can make quite a mess of things.
The easiest way to tell when the mushroom is getting ready to expose its gills is to pay close attention to the partial veil, the thin protective membrane that covers them. Initially, when the cap is completely inrolled (looking much like those old-fashioned globe-on-a-pole streetlamps), the partial veil is hidden (see photo, p. 62). As the cap starts to expand, the veil emerges as a circular, light-colored band around the bottom hemisphere of the cap.
Once the cap begins to flatten out, the partial veil gets stretched beyond its ability to expand and begins to tear, pulling away from the outer edges of the gills. Eventually, the partial veil detaches from the cap entirely, and its remnants remain attached to the stipe in a skirt-like ring, known as an annulus (see photo, p. 63). Ideally, you want to pick your mushrooms as soon as the partial veil is visible, or at the latest, before it begins to break.
Harvesting the mushrooms is as simple as grasping them at the base and twisting gently while pulling them up and away from the casing. If your fingers are small and nimble enough, you can use your clean hands to do so; if not, a pair of clean chopsticks makes a good harvesting tool. Any part of the mushroom that remains behind in the casing will rot, so take care to remove it all, down to the base of the stipe. Try not to touch the casing layer, and take special care not to damage nearby less-developed mushrooms or primordia. Sometimes, however, it is impossible to avoid disturbing or uprooting nearby mushrooms when removing another. In this case, it is better to remove these “babies” as well, rather than leaving them behind. Often, disturbing them severs their connection to the substrate and they stop growing and eventually rot. What energy the culture would otherwise have expended on these fruits will be diverted to others, so don’t worry too much about the occasional loss.
Usually, a fair amount of vermiculite will be stuck to the base of the harvested mushrooms, leaving behind a divot in the casing layer. Once you are done harvesting, simply fill these holes with fresh vermiculite, mist the casing thoroughly and return the jars to their fruiting area. During the period immediately following a harvest, increase misting frequency significantly, in order to replace the substantial amount of moisture removed from the cakes.
Each jar should produce three to five crops, or flushes, of mushrooms, with about a week of recovery time between each harvest. During the later flushes, when the nutrients of the substrate are substantially depleted, the cakes will shrink and pull away from the sides of the jar, exposing the walls of the cakes to the atmosphere and to light. Mushrooms will then begin to form around the sides of the cakes. Aside from being somewhat more difficult to remove completely from the jar, these are not a problem (this is where a pair of chopsticks comes in handy).
After the fourth or fifth flush, the jars will be pretty much fully depleted, and the number of mushrooms that form will be minimal. At this point, the cakes should be discarded, since the mycelium in them will begin to die and will eventually rot, becoming a vector for contamination.
Drying the Mushrooms & Taking Spore Prints
See chapter 12 for a variety of methods for drying and preserving your harvest, as well as making spore prints.
Cloning a Fruiting Strain to Agar
See chapter 7 for details on how to isolate a good fruiting strain.
Making Spore Syringes
For photos of this process, see p. 64.
If you have a spore print and access to syringes, you can easily make your own spore-water syringe to use in the PF method.
Materials
Sporeprint
Syringe and needle
Sterile water in a flask, sealed with foil (~15mL/per syringe)
Jet-Dry, or similar dishwashing rinse agent
Alcohol lamp
Inoculation loop
Syringe and needle
Sterile water in a flask, sealed with foil (~15mL/per syringe)
Jet-Dry, or similar dishwashing rinse agent
Alcohol lamp
Inoculation loop
1. Place 25 ml water for each syringe you will be preparing in a ½-pint jar fitted with a filter disc and lid, along with 2 drops of dishwasher rinse agent. The rinse agent can be found in most grocery stores. Look for a brand without vinegar or added scents. Its purpose here is to prevent spores from clumping and sticking to the sides of the jar and syringe.
2. Seal and sterilize for 30 minutes at 15 psi. Allow to cool completely before using.
3. To guarantee sterility, this method is best performed in a glovebox or in front of a flowhood.Wipe your work area down well with rubbing alcohol or 3% hydrogen peroxide and allow to dry.
4. Light your alcohol lamp, and heat the inoculation loop until red hot.
5. Unscrew the lid, lift it slightly from the jar, then cool the loop in the sterile water.
6. Replace the lid loosely on the flask and then use the loop to pick up some spores from the print.
7. Lift the lid, swirl the loop in the water, and then replace the lid. Repeat once or twice for good measure. Individual spores are minute; you will not necessarily be able to see them in the water.
8. Draw some spore suspension into the syringe, gently filling and emptying the syringe two or three times to insure an even distribution of spores.
9. Replace the needle cover, label the syringe with the strain and date, and place it in a clean Ziploc bag until needed. Syringes prepared in this way can be stored in the refrigerator for a month or two, but are best if used as soon as possible.
7
WORKING WITH AGAR
Preparing Agar Plates
Mushroom cultures are generally grown out and maintained on nutrified agar plates. The flat, semi-solid surface of the agar produces a radial, two-dimensional growth pattern, allowing for easy examination of a culture and the identification of and separation from any contaminants that should arise. Agar is a polysaccharide (a sugar-like molecule) found in the cell walls of certain algae. When dissolved in boiling water and then cooled, agar partially solidifies, much like gelatin. Agar itself provides no nourishment to the fungus, so a variety of nutrients are added to the medium, such as malt sugar and yeast extract. The ingredients are combined with water in a heatproof container, sterilized in a pressure cooker, and poured into Petri dishes while still liquid. One of the media most commonly used is Malt-Yeast Extract Agar, or MYA, for short. It is an all-purpose medium, one on which all species of Psilocybe will grow quite happily.
Senescence
Growers often alternate media recipes to avoid strain senescence, the degradation of a culture due to aging. Sometimes after a number of transfers between plates, a culture can begin to grow anemically, or even stop growing altogether. Senescent cultures tend to fruit poorly or not at all, and are usually discarded in favor of re-isolation of a healthy strain from spores. The causes of strain senescence are still not well understood, but it seems to occur most often when a culture is maintained on the same media recipe for a long period of time. Fungi (like humans) seem to do best when provided a variety of foods to consume, and like us they grow bored and even die when given the same thing to eat day in, day out.
To avoid senescence, it is imperative that you vary your media recipe every time you use it, which “exercises” the fungus, challenging it to produce different sets of enzymes all the time. One simple way to do this is to add small quantities of grain flour to each batch, rotating the type you use each time you pour new plates, as described below.
Occasionally, however, you might want to challenge your fungus even further, by asking it to grow on a completely novel medium. This can be particularly helpful for reviving a culture that begins to show evidence of weakening. In this case, you want to exclude all simple sugars and starches completely, and give it something completely novel to digest. (We call this recipe “Anything” Agar.) It may grow slowly on the new medium, but after a few weeks of growth, when you transfer it back to a more balanced medium like MYA, it is likely to explode with new growth. What should you feed it? Any cellulose, starch, or sugar will do, including soybeans, paper pellets, raspberry jam, peanut butter—whatever you can think of.The sky’s the limit. We have even heard of one cultivator who fed his fungi dried crickets he found at a pet store! Every so often you may find a material that your fungi refuse to grow on. If so, no problem, just try something else.
Another way to avoid strain degradation is to minimize the number of transfers of each culture that you perform. Rather than making a new generation each time you need a new culture, make many multiple copies of early generations, and store them for later use. See the section on strain storage at the end of this chapter for more details.
Malt Yeast Agar (MYA) Medium
For photos of this process, see p. 65.
22 g agar
12 g light malt extract
1 g yeast extract
1/4 tsp organic grain flour (rotate among oats, cornmeal, amaranth, rice,
millet, rye or any other starch or sugar you can think of)
5 g hardwood sawdust or wood fuel pellets
1 L tap water
8mL 3% hydrogen peroxide (optional, added after sterilization & cooling;
see below.)
12 g light malt extract
1 g yeast extract
1/4 tsp organic grain flour (rotate among oats, cornmeal, amaranth, rice,
millet, rye or any other starch or sugar you can think of)
5 g hardwood sawdust or wood fuel pellets
1 L tap water
8mL 3% hydrogen peroxide (optional, added after sterilization & cooling;
see below.)
1. Add all dry ingredients to jar, followed by the water. Be sure to use a jar that is 1.5 to 2 times the volume of media desired, so it does not boil over during sterilization. Plug the neck of the bottle with cotton wool, then wrap the opening and neck of the bottle with aluminum foil.
2. Place the jar in the pressure cooker along with the required amount of water. If you will be adding peroxide to the agar, be sure to sterilize a couple of measuring pipettes as well, wrapped in aluminum foil to maintain sterility before use.
3. Sterilize at 15 psi for 30 min. Do not cook your agar media for longer than 45 minutes, since this can cause the media to caramelize, and fungi do not grow well on caramelized sugars.
4. Allow the pressure cooker to come to atmospheric pressure, then carefully move the jar and pipettes to a glove box or in front of a flow hood while still hot. It is helpful to use several layers of clean paper towel as a potholder when moving items from the pressure cooker to the workspace.
5. When using peroxide: once the outside of the jar is cool enough to handle comfortably but still quite warm (between 120˚-140˚F), add 8 mL of 3% hydrogen peroxide, using a sterile pipette or measuring spoon. Gently swirl the medium several times in both directions to mix it in thoroughly. Take care not to over-agitate it and create bubbles, which will end up in your plates.
6. Open your sleeves of Petri dishes as directed on the packaging, and stack them right side up on your work area. Retain the plastic sleeve for later storage.
7. Working with stacks of ten dishes at a time, lift the entire stack in one hand by the lid of the bottom plate, leaving its bottom half on the bench top, and slowly pour just enough medium into the plate to cover it completely. Replace the stack, and repeat with the plate above it, until complete. One liter of medium should be enough for 20-30 100 mm standard Petri dishes. Try not to agitate the mixture as you pour. If there are solid particles at the bottom of the flask, leave them there; any available nutrients should be in solution, and you want the media in your plates to be clear enough to see through.
8. If you find that the agar begins to solidify before you complete pouring it, it is often helpful to keep the jar in a shallow pot of hot (~150˚F) water when not in use.
9. Stack the completed plates in one column, and loosely replace the sleeve they came in to allow every plate in the stack to cool slowly and evenly, minimizing condensation on the upper plates (condensation can make the agar difficult to see, and can become a vector for contamination). A similar effect can be achieved by covering each stack with a clean coffee mug or heavy glass half-filled with hot water.
10. Allow plates to cool overnight.
11. Peroxide plates can be left for several days in a cool draft-free spot to further drive off any remaining condensation. Lay them out in stacks of two or three, loosely covered with a few sheets of clean waxed paper. Plates without peroxide should be placed in sleeves as soon as they have cooled.
12. Slide the plastic sleeve back over the plates and tape it shut tightly with clear packing tape. Store them agar side up (to further minimize condensation) in a cool and dry place until needed.
Here’s a recipe for an “anything goes” agar medium, for when you want to really put your mycelium to the test:
“Anything” Agar Medium
20 g of anything
22 g agar
1 L tap water
8 mL 3% H2O2 (added after sterilization)
22 g agar
1 L tap water
8 mL 3% H2O2 (added after sterilization)
1. Grind the material to a fine powder if it is not already, or puree it with the water in a blender if that is more practical.
2. Follow instructions for MYA preparation as described on pp. 95-97.
Care of Petri Dishes & Cultures
When making transfers, lids should be removed for as brief a time as possible, and held directly above the plate to keep contaminants out.
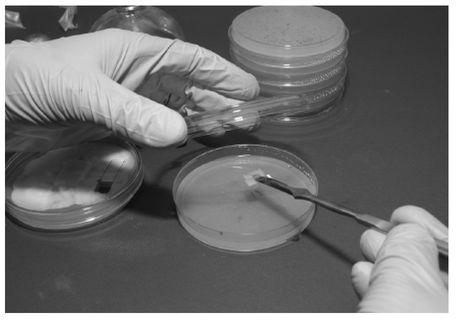
Always hold the Petri lid directly over the plate when accessing it to minimize opportunities for contamination.
Cultures should be stored agar side up as well. However, it is a good idea to give new transfers a day or so to grow out onto the new plate before turning them upside down, or the transferred material might not adhere to the fresh agar surface. Culture plates should be wrapped around their edges with several layers of parafilm.

Plates stored agar side up, wrapped in parafilm.
Spore Streaking
Unless you are using the PF Tek to grow P. cubensis mushrooms or collecting fresh specimens from the wild, you’ll need to start your cultures from spores to isolate a pure fruiting strain.There are two methods you can use to start mushroom cultures from spores on agar. In the traditional method, a sterile inoculating loop is used to pick up a tiny amount of spores from a print, and then streaked across an agar plate. Keep in mind that you cannot use Petri dishes containing peroxide for this purpose, since the spores would be killed by it.
Alternatively, in an innovative method devised by Rush Wayne and described in volume two of his book Growing Mushrooms the Easy Way, spores are germinated on sterile cardboard discs.This method provides several distinct advantages over the traditional method. The small size of the discs and the narrow openings of the test tubes help keep contaminants out of cultures that are unprotected by peroxide. In addition, it is a fairly rapid system: the discs are quickly colonized and can then be placed directly onto peroxidated agar. Finally, because the disc acts both as the substrate and as the tool used to lift spores from the spore print, it provides for a very efficient transfer, which is especially helpful when the spore print is faint and light on spores.
Using a hole puncher, small discs are cut from thin, flat cardboard (the gray sheets found at the back of pads of paper are perfect).These are moistened slightly, placed in a jar and sterilized along with test tubes containing 5-10 drops of a malt-yeast extract solution. When cool, the discs are used to pick up a small amount of spores, and then dropped into the tube, where they absorb the malt solution. In time, the spores germinate and when the tiny discs are fully colonized, they are transferred to peroxide agar plates.
Agar Spore Germination
This method is identical to the one used when making spore-water syringes (chapter 6), except that here the spores are transferred to peroxide-free agar plates instead of water.
Materials
Spore print
Peroxide-free agar Petri dishes
Inoculating loop
Alcohol lamp
Parafilm
Peroxide-free agar Petri dishes
Inoculating loop
Alcohol lamp
Parafilm
1. In your glove box or flow hood, heat the inoculation loop in the alcohol lamp until it glows red-hot.
2. Lifting the lid of the first Petri dish with your other hand, press the tip of the loop to the center of the agar to cool it (this also places a thin film of agar on the loop, which will help the spores to adhere to it).
3. Cover the plate and then use the loop to pick up a small amount of spores from your print.
4. Streak these across the Petri dish in an S-shaped motion and then close the plate.
5. Re-sterilize and cool the loop before streaking each plate.
6. After inoculation, wrap the edges of each plate with parafilm, mark them with any relevant information, and incubate agar side up.
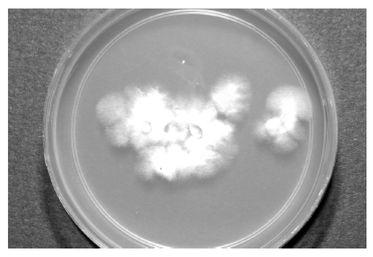
Spores germinating on an agar plate.
Cardboard Disc Spore Germination
For photos of this process, see p. 66.
Materials
Spore print
Cardboard discs
½-pint jar and lid
Screw-capped test tubes or vials (2 or 3 per spore print)
Malt-yeast agar solution (1 tsp malt and a tiny pinch of yeast extract in 100
mL water)
Pipette or eyedropper
Tweezers
Alcohol lamp
Parafilm
Cardboard discs
½-pint jar and lid
Screw-capped test tubes or vials (2 or 3 per spore print)
Malt-yeast agar solution (1 tsp malt and a tiny pinch of yeast extract in 100
mL water)
Pipette or eyedropper
Tweezers
Alcohol lamp
Parafilm
1. Place cardboard discs in ½-pint jar, along with 1-2ml water, and seal. Place 5-10 drops of malt solution into test tubes and lightly seal. Sterilize the jar and tubes for 15 minutes at 45 psi and allow to cool completely.
2. Place all tools and materials in your glove box or flow hood.
3. Heat the tweezers in the alcohol lamp until hot, and allow to cool.
4. Open the jar and use the tweezers to remove one disc. Cover jar.
5. Lightly touch the edge of the disc to part of the spore print.You should be able to see the black spores adhering to the disc.
6. Open a test tube and drop the disc onto the bottom of the tube.
7. Repeat 3-5 times per tube.
8. Create at least two tubes of discs for each spore print.
9. Seal the tubes with parafilm and incubate.
10. When the spores have germinated and the discs are fully colonized, transfer a few to individual peroxide-containing agar plates.
Incubation
Inoculated culture plates and spore discs should be incubated in a warm, draft-free location, in the 75-85˚ F range. If the temperature in your home is consistently within this range, then it is sufficient to simply store them in a clean box. If not, an incubator like the one described in chapter 6 will insure healthy and rapid colonization.
Tissue Transfers (Cloning)
Fresh, clean mushrooms, either fruited from a multispore culture (from a PF Tek jar, for example), or collected from the wild, can also be used to start an agar culture. In this case, the resulting culture should be a single strain and should display the same characteristics as its parent. Since it is genetically identical to the strain from which it was isolated, it is considered a clone, and this technique is known as cloning. Because of this, we generally look for the healthiest specimens in a population to clone, with the hope of isolating a strain that will provide consistent and dynamic fruitings with each use. Good characters to look for in a parent include early, large, or dense fruits, and any that have a healthy look overall.
Isolating a single, fruiting strain is as simple as picking the choicest specimens from your crops and culturing them on agar. The mushrooms are broken or cut open in a glove box or flow hood, and a small piece of sterile mycelium is removed from the inside of the stipe or the area of the cap just above the gills and placed on a fresh agar plate. After a short incubation period, the mycelial fragments grow out onto the plate and can then be subcultured.
Often however, for unknown reasons, clones taken from the same parent mushroom do display differing mycelial characteristics.21 For this reason, we make numerous (four or more) cultures from each one, and save only the best resulting cultures for further use. Looks can be deceiving and strains may not perform as predicted by their appearance, so we clone as many different specimens as time and space allows, in order to enhance our chances of success in the long run.
Because they have never been exposed to the external environment, the cells on the inside of a mushroom should be sterile (i.e., uncontaminated). To guarantee sterility, however, you should always try to clone mushrooms as soon as possible after picking them. If you cannot use them right away, you can store them in the fridge in a clean Tupperware container lined with a fresh paper towel for a few days, but not much longer.
Unlike when streaking spores, peroxide in the agar media will actually enhance your chances of successful cloning, since it provides an additional layer of protection from contaminants. Therefore, we highly recommend using peroxidated agar whenever doing tissue cultures, unless you find that the species or strain in question does not tolerate it.
Tissue Transfers
For photos of this process, see p. 67.
Materials
Mushroom(s)
Petri dishes (with peroxide)
Scalpel
Alcohol
Alcohol lamp
Cotton balls or paper towel
Petri dishes (with peroxide)
Scalpel
Alcohol
Alcohol lamp
Cotton balls or paper towel
1. Mushrooms for cloning should be thoroughly cleaned of any loose casing material before use. This work should be completed away from the work area if possible.
2. Preferably working in your glove box or flow hood, wipe the outer surfaces of the mushroom with an alcohol-soaked cotton ball.
3. Sterilize the scalpel in your alcohol lamp. Holding the mushroom at the base of the stipe, squeeze it very gently between your thumb and forefinger.You should be able to split it along the centerline, and then peel the two haves of the mushroom apart lengthwise, all the way through the cap, if possible. If it is a very small specimen, or does not split easily, you can instead use the scalpel to cut it open. Try to avoid letting the knife directly contact the area you want to clone from, since it could introduce contaminants present on the outer surfaces of the mushroom into your culture.
4. Re-sterilize the blade after each use.
5. With your Petri dishes ready, cut a small piece of mycelium from a suitable area of the stipe or cap (generally at the thickest, most densely packed location.) It should be as large a chunk as possible, ideally from 3-8 millimeters wide and long. Be especially careful to avoid cutting all the way through to the unsterile outer layers of the mushroom.
6. Spear the fragment mycelium lightly on the tip of your scalpel. Lifting the lid of the Petri dish in your other hand, place the fragment on the center of the agar and then close the plate. (Sometimes the fibrous nature of the mycelium can cause it to stick to the tip of scalpel blade; if so, try cutting through the fragment, pushing it down into the agar as you do so.)
7. Repeat with three or more plates per mushroom.
8. Seal the plates with parafilm, mark them appropriately, and place them in your incubator. Store them right side up until they begin to grow out, and then reverse them as usual.
You should begin to see growth within a few days to a week. At first, the fragment will become uniformly fuzzy, as its cells begin to divide again; eventually the mycelium will fan out from where it contacts the agar to colonize the entire plate. Healthy cultures should be subcultured as soon as possible (see below), using only clean mycelium from the leading edge of new growth, since the original tissue might harbor hidden contaminants.
Agar-to-Agar Transfers (Subculturing)
To make agar-to-agar transfers, a small piece of healthy looking mycelium from the edge of a culture is cut out with a sterile scalpel and placed on the center of a new plate. Growing cultures should be used or subcultured before the mycelium comes within 1 centimeter of the edge of the plate, since the outer edges of plates may harbor contaminants, which would be hidden beneath the advancing mycelial front, only to emerge upon transfer to a new medium.
If the culture is a pure strain, any portion of the advancing edge may be subcultured. If it is a multi-strain culture (such as that arising from a multispore inoculation), then you will want to select mycelium with the desired characteristics.The appearance of two or more mycelial types within a single culture is known as sectoring. The visual appearance of a healthy strain varies somewhat from species to species, but dense, rhizomorphic growth is a good indication of general health for all Psilocybe species. Avoid sectors with slow growing or wispy-looking mycelium, which are less likely to produce a fruiting strain.

A fully colonized agar plate ready for transfer.
When making a transfer, always place the agar wedge face down on the fresh plate. This serves two important functions. First of all, it places the mycelium in direct contact with the agar, promoting rapid colonization of the new plate. Second, by sandwiching the mycelium between two layers of peroxide-containing agar, any contaminant spores or bacteria that are hiding on the mycelial surface will be killed.
Agar-to-Agar Transfers
For photos of this process, see p. 68.
Materials
Agar culture(s)
Sterile agar plates
Scalpel
Alcohol lamp
Parafilm
Sterile agar plates
Scalpel
Alcohol lamp
Parafilm
1. In a glove box or flow hood, remove any parafilm from the outside of your healthy culture dish.
2. Heat the blade of the scalpel in your alcohol lamp until it glows, then cool it in a fresh Petri dish.
3. Holding the lid of the original culture dish slightly ajar, cut squares or wedges of agar and mycelium from the desired portions of the plate, from ½ to 1 centimeter wide. For simplicity’s sake, you may cut more than one wedge at a time.
4. To make the transfer, remove the lid of the original culture plate entirely.With the knife in one hand, lift the lid of the clean plate slightly to one side, spear one wedge of agar from the culture plate on the tip of the knife and place it on the center of the new plate, mycelium side down.
5. Repeat on all plates, seal and mark appropriately, and place in the incubator, upside down as usual. (Wedges of agar adhere well to fresh agar, allowing the plates to be inverted immediately.)
Contamination
Contamination is inevitable in mushroom cultivation work. One of the benefits of working with the two-dimensional surface of agar plates is that contaminants are readily observed and isolated from healthy cultures. Plates showing any sign of contamination should be removed from the growing area and disposed of immediately.
Occasionally, it may be necessary to try to “rescue” a contaminated culture (if you always do multiple identical transfers and keep clean work habits, such occasions should be extremely rare.) In this case, you should always move the culture away from its contaminants to a new plate. If you try to remove contaminants from an agar dish by cutting the invaders from the plate, you will very likely only spread the contamination further.
Because mold spores are so easily disturbed, it is exceedingly difficult to avoid moving contaminants along with your culture to new plates, and may take several transfers before they are fully eliminated.

Mold colonies growing at the edge of a mushroom culture plate. In order to rescue this culture, the mushroom culture must be carefully transferred to a clean plate.
Diagnosing the Sources of Contamination on Agar
You can often determine the source of contaminants on agar by observing the pattern of the contamination on the plate.
1. If contamination appears before the plates are used, it can be an indication of inadequate sterilization of the agar, poor sterile technique during pouring or storage of the plates, or an insufficient concentration of peroxide in the medium.
2. If signs of contamination occur on the extreme edges of the plate, either in individual colonies or in a complete ring, it can indicate that non-sterile air had been drawn into the plates as they cooled. To prevent this, let the agar cool sufficiently before pouring it, and cover the plates with their plastic sleeve immediately after filling them.
3. Contamination originating at the inoculation point is an indication of either a contaminated parent culture or insufficient sterilization of the knife or inoculation loop. Examine cultures to be transferred carefully before using them, and avoid using any that are suspect. Always heat inoculating tools until they glow red-hot.
4. Bacterial contamination appears as slimy, shiny, translucent circular colonies, often white, pink, or yellow in color. Bacteria thrive in wet environments, and are easily spread onto plates with excess condensation on their lids.Always wait until agar has cooled sufficiently before pouring, let plates cool slowly in their plastic sleeve, and store them agar side up.
Long-Term Strain Storage
Once you have isolated a healthy fruiting strain, you will want some way to propagate this same strain for a long time, so that you don’t have to constantly repeat the isolation process. Cloning from plate to plate over and over again will eventually cause the strain to senesce, even if you alter your agar recipe consistently, as we recommend. Therefore, you always want to use cultures that have been subjected to as few transfers as possible. To do this, you should create a “master” culture of any strain you consider worthy of propagation, as soon you identify it. The master culture is then placed into refrigerated, long-term storage, and subcultured as needed.
Cultures that are stored at standard refrigerator temperatures (~38˚ F) enter a state of suspended animation, and can be restored by simply subculturing them to a fresh plate. After a short recovery period, the culture will resume normal growth.
We recommend storing cultures on sterilized paper pellets in test tubes22. Strains stored on agar can die out unexpectedly, perhaps owing to the high sugar content of the media. The nutritional content of paper is minimal, but apparently sufficient to keep the culture healthy for long periods. The narrow mouths of test tubes are ideal for minimizing condensation and contamination, and their small size allows for easy storage. However, if you do not have access to test tubes, you can use ½-pint mason jars or other similar small autoclavable containers. After transferring the culture to the tube and allowing it to grow out, the tubes are placed in a secondary container (such as a Ziploc bag) and stored in the fridge. Strains kept this way can remain viable for many years, but it is a good idea to retrieve cultures periodically (once every year or two) by subculturing each to a plate, then returning to fresh paper for further storage.
The effects of peroxide on cultures stored for long periods are not well known and therefore we leave it out of our storage media.
Paper Pellet Storage Medium
For photos of this process, see p. 69.
Materials
Paper pellet cat litter
Tap water
Test tubes or other suitable containers
A funnel
Tap water
Test tubes or other suitable containers
A funnel
1. Moisten paper pellets to field capacity.
2. Load into tubes, ⅓-½ full. Be careful to remove any bits of medium from the outside of the tubes. Seal loosely.
3. Place the containers in your pressure cooker and sterilize for 30 minutes at 15 psi. Jars can be stacked in layers, while test tubes should be placed in a rack or a metal can to keep them vertical.
4. When the cooker has returned to atmospheric pressure but is still warm to the touch, open it, and carefully transfer the containers to your glove box and allow to cool.

Paper pellet culture tubes beginning to be colonized.
Inoculating Storage Tubes
Paper pellet tubes are inoculated similarly to Petri dishes. Because the cultures lack peroxide, and they are meant to be stored for the long term, you should take extra care to avoid introducing contaminants when doing so, following all the usual precautions. In addition, you should sterilize the neck of the slant tube each time it is opened, by rolling it in the flame of your alcohol lamp.
1. Flame the scalpel and the neck of the open tube.
2. Cut a small piece of agar from a healthy culture, and place it in the sawdust tube. Since test tube necks are too narrow to allow the knife to reach, it is easier to hold the tube horizontally, place the wedge on the upper wall of the tube, seal it, and then gently knock it down onto the sawdust.
3. Seal the container, wrap the cap or lid with a band of parafilm, and mark it appropriately.
4. Incubate until the paper is fully colonized, then place the tubes into a secondary container such as a freezer bag or Tupperware container and refrigerate.
Retrieving Cultures From Storage
To retrieve a culture from its storage container, return the culture to room temperature for 48 hours, and transfer (under the usual sterile conditions) a small chunk of mycelium-covered paper from the storage container to a fresh, peroxidated agar Petri dish.
8
WORKING WITH GRAIN
Grain Spawn Preparation
Grain spawn is made of cooked whole grains with small amounts of calcium carbonate and calcium sulfate added. The calcium carbonate serves as a pH buffer, while the calcium sulfate keeps the individual grain kernels from sticking together; both provide mineral nutrition to the fungus. To ensure even cooking and thorough sterilization, the grain is boiled briefly, soaked overnight in hot water, then drained, loaded into containers, and sterilized.
Many grains can harbor bacterial endospores. These highly resistant structures are designed to withstand extreme environmental conditions, allowing them to survive sterilization. Soaking the grain overnight forces these endospores to germinate, at which point heat will easily kill the live bacteria.

Raw white wheat berries.
Almost any largekernelled, organic whole grain will do, including wheat, rye, corn, milo, or millet. We use white (soft) winter wheat because it is easy to find, cooks nicely, and tends to be low in bacterial endospores.
Grain Spawn Recipes
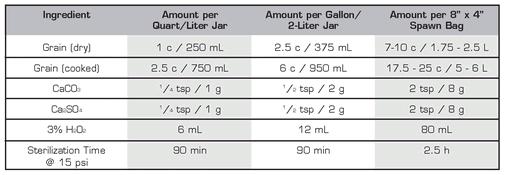
1. Be sure to use a large enough pot, as the grain will more than double in volume when cooked (three times the final volume is a good start). Fill it with at least twice the volume of water as cooked grain desired, and then bring the pot to a full boil on your stove. Carefully pour in your dry grain, and bring the pot back to a full, rolling boil.
2. Hold it there for 10 minutes, turn off the burner, and allow the pot to sit, covered, for at least 8 hours (but no longer than 16). At this point, it should have nearly doubled in volume and will be fully hydrated (to test, crush one grain between your fingers, it should be completely soft on the inside.)
3. Drain thoroughly in a colander. If the grains are at all sticky after draining, rinse in several changes of cold water and drain well.
4. Load the grain it into your containers, along with the appropriate amounts of calcium carbonate and calcium sulfate. Seal, shake well, and load into your pressure cooker, making sure to leave as much space as possible between individual containers. (Be sure to also include a pipette or other autoclavable measuring container for measuring out your peroxide later.)
5. Sterilize for the appropriate amount of time.
6. Allow to cool completely before using, or overnight if necessary. Leave the jars inside the sealed pressure cooker until ready to inoculate.
Loading jars with cooked grain.

Agar-to-Grain Transfers
For photos of this process, see p. 70.
Agar cultures are used to inoculate small quantities of grain, generally in quart-sized jars, which can then be fruited or used to inoculate larger containers of grain. One fully colonized plate can be used to inoculate as many as 6 quart jars. Peroxide is generally added to the grain at the time of inoculation to provide additional protection from contaminants, at the rate of 6mL for every 2-3 cups of cooked grain.
Materials
Sterilized grain jars
Agar culture
Scalpel
Alcohol lamp
3% H2O2
Sterile 10mL pipette or measuring spoon
Agar culture
Scalpel
Alcohol lamp
3% H2O2
Sterile 10mL pipette or measuring spoon
1. Open the pressure cooker and place all the jars in your glove box or flow hood.
2. Loosen the cap of each jar completely without removing it, then pipette or measure the desired volume of H2O2 into each jar, lifting and replacing the lid with your other hand. Seal the jars tightly.
3. Shake each jar well to thoroughly separate the grains and distribute the peroxide, and then shake it again to one side of the jar, leaving a steep slope of grains. Loosen the lid fully but do not remove it. Place the jar back on the work area, taking care not to disturb the grain inside.
4. Repeat with the remaining jars.
5. As with agar-to-agar transfers, sterilize your knife and cool it in a blank agar plate (or allow it to cool gradually).
6. Open the culture plate and cut it into wedges or squares (at least two for every jar). Do not cut into the original parent culture at the very center of the plate.
7. Spear one wedge on the end of your scalpel, close the plate, lift the lid from the first jar, and tip the agar wedge to the bottom of the hill of grain, preferably mycelium side down (touching the grain).You may tap the handle of the knife onto the outer rim of the jar to dislodge it from the knife if you need to, but do it gently so as to avoid contamination.
8. Repeat with another wedge, place the lid back on the jar, and then move on to the next jar.
9. Once all jars have been inoculated, tighten their lids, and gently knock back down the hill of grains to cover the agar wedges completely. Do not shake the jars at this stage; though many mushroom cultivation methods recommend doing so, we have found that first giving the mycelium a chance to leap off the agar onto the grain before shaking results in healthier cultures.
10. Label the outside of the jars appropriately with a permanent marker and place into your incubation chamber or area.
Syringe Inoculation of Grain
You can also use PF-style spore water syringes to inoculate grain. Since spores would be killed by hydrogen peroxide, it must not be used here.The method is simple:
1. After the grain has been sterilized and the jars have cooled to room temperature, transfer them to the glove box or flow hood.
2. Loosen but do not remove the lid of each jar.
3. Remove the cover from the syringe, wipe the needle with an alcohol-soaked cotton ball, and then hold the tip of the needle in the flame of your lamp until it just begins to glow red. Allow it cool for a few seconds before using.
4. Momentarily open one jar at a time as you inject a few milliliters of spore suspension onto the grain.
5. Seal, shake, and incubate.
Incubating Your Jars
Because of their large size and awkward shape, you will probably not have enough room in your incubator to store all of them. Unless you want to build a few incubators, or some other kind of heated system, you can simply place the jars in a warm, draft-free space, such as on a closet shelf. So long as the temperature is between 65-80˚ F they will grow without additional heat, if somewhat slower at the lower temperatures. Actively growing mushroom mycelium gives off heat as it consumes the substrate.There is generally enough activity in a quart jar to raise its temperature above ambient by several degrees. For this reason, it is better to err on the low side. To make sure they don’t overheat, avoid placing the jars where the temperatures are much higher than 80˚.
Shaking Grain Jars
Actively growing grain cultures are shaken periodically to encourage rapid and even growth. A few days to a week after inoculation, the mycelium should have leapt off of the agar wedges and begun to colonize the surrounding grain. Once this expanding sphere of mycelium has reached an inch or so in diameter, it is ready to shake. Gently agitate the jar to break up the cluster of grains and redistribute them.
As the jars become more and more colonized, the individual grains become bound together by the mycelium and can be difficult to separate. If you must use force to break up a tightly bound jar of grain, do not bang it against the palm of your hand, or any other part of your body, for that matter. Jars, particularly those that have been through many sterilization cycles, can often contain hidden stress cracks and can shatter unexpectedly. Using the palm of your hand to shake a jar could result in a serious injury. Instead, bang the jar on a thick clean towel supported by a firm pillow, or on the edge of a partially used roll of 2-inch duct tape placed on a sturdy tabletop.
Jars should be shaken twice, at approximately 5% and 50% visible colonization, or about once a week until all kernels are fully colonized. After shaking for the first time, the mycelium around the colonized grains will have been broken into nearly invisible fragments. At this point, it might seem that there is no life in the jar at all, but within a day or two you should begin to see the mycelium recover and start to colonize the grain at many more individual points than before (photos of grain jar colonization, p. 71).
Understanding Contamination on Grain
Examine your grain jars carefully and often for signs of contamination. Pay particular attention after shaking; often seemingly clean jars will reveal hidden contaminants several days later. If the mushroom mycelium does not recover, or does so only very slowly, it could be an indication of bacterial contamination. Bacterial contaminants often appear as wet spots or bubbles surrounding the grains, or on the inner surface of the jar. Another mark of bacterial contamination is a rotten apple or sour, fermented odor; this is often detectable by giving the jar a sniff through the filter disc. Mold contaminants on grain, with their highly pigmented spores, are generally easy to spot (see p. 71 for photo of grain contamination).
It is especially important when working with grain spawn to segregate contaminated containers from clean ones as soon as possible. Grain is so nutritious that a single mold spore can colonize an entire jar in a matter of days and produce literally billions of spores in the process. Opening such a jar can create a huge cloud of spores, contaminating an entire room for a very long time. Some growers go to the trouble of pressure-cooking their contaminated containers before opening them, in order to sterilize the contents before they can escape into the lab. While that may be overkill, at the very least you should dispose of the contaminated grain and thoroughly clean the container that held it in a remote location before reusing it, and be sure to shower and launder your clothes before doing any further culture work.
Grain-to-Grain Transfers
Once grain containers are fully colonized, they should be used as soon as possible.The viability of spawn declines steadily after a week or two, along with your chances of success.
Smaller containers of colonized grain can be used to inoculate new ones, large or small. In this way, a small amount of spawn can be expanded to greater amounts. One jar can inoculate up to ten new jars of similar size, or up to four larger containers, such as spawn bags. Each “generation” of spawn can be used to create another one, though it is best to limit such transfers to no more than three generations to avoid strain senescence.
Keep in mind that quart jars of grain can be used to directly inoculate fruiting substrates, or can even be fruited themselves (at least in the case of P. cubensis.) Grain-to-grain transfers are only required when you need to further expand your amount of spawn.
From each collection of jar cultures, select only the few healthiest specimens to transfer. In any population of cultures, there will be some that naturally appear healthier than others. Reject any that show even the slightest hint of weakness, such as slow growth, wet spots, or grains that remain uncolonized even after shaking multiple times. Shake the best jars one more time, and allow them to incubate for another day or two to allow one last opportunity for contaminants to reveal themselves. Healthy cultures should recover within this time, with each grain showing good mycelial growth; any other result should qualify a culture for the reject pile.
Grain-to-grain transfers are performed more or less like agar transfers: After adding peroxide to the sterile grain jars, a small amount of colonized grain is free poured into them.The exact amount of spawn added depends on the total number of new containers to be inoculated, and should be evenly divided between them. The newly inoculated jars are then sealed, shaken, labeled and incubated as before.
Bags and Other Large Spawn Containers
Many growers use autoclavable spawn bags when preparing spawn in quantities greater than one quart. This is for a number of reasons. A single standard-size bag (18” x 8” x 4”) can hold ten times as much grain as a single quart jar, and bags take up far less space inside a pressure cooker or incubation area.The flexibility of the bag also allows for easy manipulation and examination of its contents. Shaking of jars larger than one quart is awkward, while the grain inside a bag is easily broken apart and redistributed.
Bags are always inoculated from jars or other large containers of grain, never from agar. In order to colonize the large volume of substrate in the bag quickly enough to win the race against contaminants, you need to use a high percentage of inoculum in each. Growers usually use a cup or so of colonized grain per bag, or 2-4 bags per quart jar of inoculum.
One of the drawbacks of using bags is that their large size makes it difficult to keep contaminants out and maintain sterility. Even a single bag takes up a lot of room in a glove box, and working with more than one at a time is nearly impossible. For this reason they are generally inoculated in front of a large HEPA flow hood. Even if you do have access to an adequate flow hood, we recommend using peroxide in all of your grain spawn bags, to further protect them.
Spawn bags are sealed using an impulse sealer across the top of the bag. Two or three passes a few millimeters apart insures an adequate seal.
Whenever using larger containers of spawn, whether bags or jars, it is important to avoid excessive moisture inside the containers. Small containers of grain can tolerate a slight excess of water in them; a few tea-spoons of moisture will eventually be taken up by the mycelium and pose no problems. With larger amounts of grain, however, such excess can quickly add up; pooled moisture can suffocate the mycelium and act as a vector for contamination. This is the reason for the substantially greater quantities of gypsum and calcium carbonate for larger containers given in the recipes above.
There are several steps you can take to avoid overly wet culture containers. First of all, do not overcook your grain (this is why we soak rather than boil it.) Second, allow the hydrated grain to drain thoroughly before using. Finally, if after preparing your containers, but before sterilization, they still seem too wet, add small amounts of additional gypsum to absorb the excess moisture. Properly prepared grain should be practically dry to the touch, but still moist on the inside. This is especially important when preparing bags of grain, which receive a larger percentage of peroxide than jars do, owing to their increased risk of exposure to contaminants.
Loading and Cooking Grain Bags
1. After filling bags, press out all the remaining air in the bag, and fold the flap back and under the bag, leaving the filter patch facing out.Grain bags ready for sterilization. The flap should be folded under the bag with the filter patch facing out.
2. Layer the bags in the pressure cooker in such a way that the filter patches on each remain exposed, and leave as much space as possible between bags. Use a liner or trivet to lift the bags off of the bottom of the cooker, and be especially careful that they do not touch its external walls or they will melt. If you are using peroxide, pressure-cook a graduated pipette or cylinder (A cylinder is preferable in this instance, since it can hold the full 80 mL of 0.0015% hydrogen peroxide used in each bag.) Add a healthy amount of water to the bottom cooker and pressure cook for 2.5 hours at 15 psi.Grain bags and a graduated cylinder loaded into a pressure cooker for sterilization.
Innoculating Grain Bags
For photos of this process, see p. 72.
1. After they have cooled completely, move the bags to your workspace.
2. Shake the culture jar to break up the colonized grain within.
3. Measure out 80 mL of peroxide, quickly open one bag, pour it in, and fold the flap closed to keep out airborne contaminants. Fold the flap over itself several times, and then, holding it tightly closed in your hand, shake the bag lightly to thoroughly distribute the peroxide, and leave it closed on the workspace.
4. Repeat with the remaining bags.
5. Open each bag individually, pour in the desired amount of colonized grain, fold back the flap, and repeat with the remaining bags.
6. Seal each bag with the impulse sealer. It is a good idea to do at least two swipes of the sealer with a few millimeters of space between them.
7. Shake each bag thoroughly and mark the outside of each with relevant culture information.
Incubating Larger Containers

A fully colonized grain spawn bag.
As mentioned earlier, growing fungal cultures generates heat as they digest their substrates. With very large containers, the amount of heat can be substantial, and it is important not to let them overheat during incubation. For this reason, you should allow them to incubate in a relatively cool spot, somewhere in the 65-75˚F range.
Bags should never be allowed to touch one another; leave at least 4 inches of space around each one to allow sufficient air circulation and dissipate as much heat as possible.
9
FRUITING CONTAINERS
Once you have a fully colonized substrate, you need to place it in a suitable container to be cased and fruited. The container you choose depends on the amount of substrate to be fruited, and can range from a small aluminum-foil bread pan to a large plastic bus bin.You should aim for a substrate depth of 2-3 inches when fruiting smaller quantities of grain, and as much as 6 inches for larger quantities. Aside from size, there are certain features to consider when choosing a fruiting container. It should be made of a material that is sufficiently rigid to hold the substrate in place as it colonizes, and it should be thoroughly opaque, allowing you to restrict light to the surface of the casing soil alone. In addition, the total depth of the container should ideally be no more than twice the substrate depth to allow for easy gas exchange when you open the container for misting.
Some growers use deeper, fully enclosed containers such as plastic storage bins with snap-on covers to create a humid environment. While this does work, it requires cutting or drilling holes in the sides of the container to provide gas exchange, and it means that at least the top must be translucent to allow light to fall on the casing surface.We instead prefer to use shallower, opaque containers, which are then placed inside the moist, well-lit environment of a fruiting chamber.The fruiting chamber can be as simple as a clear plastic bag perforated to allow gas exchange, placed near a sunlit window, or as complex as a multi-tiered shelf unit that holds many containers, fitted with timed fluorescent lights and a humidifier. See the color section, page 73 for photos of several suitable fruiting containers.
The two types of fruiting containers we use most often are plastic dishwashing tubs, 11.5” x 13.5” x 5” deep, which hold 4-8 quart jars or one bag of grain, or, for larger quantities of substrate, plastic bus bins, 20” x 15” x 7” deep.The smaller dishpans are sold in hardware and kitchen supply stores, and the bus bins can usually be purchased at restaurant supply warehouses. Always use dark colored, opaque containers to protect the substrate from premature exposure to light.
The Humidity Tent
The cased containers must be kept in a humid environment to prevent rapid loss of moisture from the casing and substrate. Unlike many other cultivated mushrooms that require very high levels of relative humidity (90%-100%), we have found that Psilocybe cubensis does just fine with much lower levels (down to 70%). As long as the container is placed in an enclosure that is sufficiently small, and the casing soil is kept well watered, enough water will wick into the immediate environment to keep the mushrooms happy.
Smaller trays or tubs can simply be placed into clear plastic bags and tied shut. Holes should be punched or cut in the top and sides of the bag to allow for gas exchange, with four or five ½-inch holes per square foot. (Several online mushroom suppliers sell pre-perforated bags precisely for this purpose.) The container should be removed from the bag during misting to help displace accumulated carbon dioxide.The bags should be large enough to accommodate the growing mushrooms, which will extend as much as 8 inches above the top of the casing soil.

A simple plastic humidity tent over a small tub of cased grain. Note the holes in the tent for gas exchange.
Larger single containers can be placed inside inverted clear plastic storage tubs, with holes drilled in their sides for gas exchange, or placed on a tented shelf unit. A number of garden supply catalogs and online retailers sell “grow racks.”These are three-or four- tiered lightweight racks enclosed inside a zippered plastic tent to maintain humidity. When combined with a timed lighting system these make excellent self-contained mushroom growing enclosures. Each shelf can hold several smaller containers or one bus tub, with plenty of room between each shelf to house the developing mushrooms.

A four-tiered grow rack.
Humidity Levels
Small fruiting containers should go into perforated plastic bags, single larger ones should go into large bags or clear tubs, and multiple larger ones can be placed on an enclosed grow rack. As long as the size of the humidity chamber is closely matched to the amount of substrate it contains, the moisture levels within should be fairly easy to maintain with a once- or twice-a-day hand misting. When the casing soil is well watered, it should wick enough water into the immediate environment to keep the growing mushrooms happy. If the air in your growing area happens to be particularly dry, you may need to resort to a supplementary humidification system. In this case, at least for the small-to-medium scale grower, the best choice is a “Cool Mist” (impeller) style humidifier, which does not use heat to create humidity and therefore won’t unnecessarily raise the temperatures of your cropping area. These are cheap and readily available at most larger pharmacies and department stores.

A “Cool Mist” style humidifier.
We have seen other growers construct complicated tubing systems for pumping moist air from a humidifier into a grow rack. This may be necessary when fruiting large numbers of containers at one time, but in general the humidifier itself can simply be placed on one of the shelves along side the trays. Condensation will build up on the walls of the enclosure, so it is a good idea to place a large tray beneath the rack to keep moisture from falling directly onto the floor. Empty and clean this tray often to avoid mold buildup.
Lighting
Your lighting setup should also be scaled appropriately to your fruiting area. Psilocybe mushrooms are unlike plants in their lighting requirements. They use light only to stimulate growth, not as an energy source, and they only require short daily periods of it to fruit successfully. A good rule of thumb is that if the space is lit sufficiently to see well, it should support fruiting just fine. A few PF Jars or a single tub will need little more than a south-facing window or good ambient electrical lighting. Larger grow racks will need a built-in lighting setup. We have found that 15-20 watt compact fluorescent bulbs work reliably well, and use very little energy. However, they should be mounted outside the grow chamber (perhaps mounted to an adjacent wall) to minimize heating and reduce the risk of an electrical short circuit. Depending on the size and number of containers in the enclosure, it may be necessary to mount lamps at several locations in order to avoid casting shad-ows on the cultures. Electric lighting systems should also be put on timers, set to illuminate the space for 8 hours per day.

Compact fluorescent lamp clamped to the outside of a tiered grow rack.
10
CASING SOIL
Many cultivated mushroom species, Psilocybe cubensis included, will fruit abundantly only if the substrate is covered in a soil-like layer known as a casing layer. Casing soils are generally made up of non-nutritive materials with high water-holding capacities, such as peat moss or vermiculite, along with gypsum and calcium carbonate.The casing layer serves a number of important functions for the developing mushrooms. Because of its high water content, the layer helps to keep the substrate from losing its moisture to the atmosphere.This creates a humid microenvironment within which the fragile primordia can form, and acts as a water reserve for the thirsty mushrooms to draw upon as they grow. Since the casing layer takes up and releases water like a sponge, it also allows a grower to easily maintain a bed at its optimum moisture level while minimizing the risk of waterlogging the substrate and drowning the fungus. In addition, the moisture level is often easier to “read” on the particulate casing layer than it is on bare colonized substrate, simplifying the process of humidification.
Many casing soil recipes incorporate mineral salts such as chalk and gypsum. Peat moss is somewhat acidic, and mushroom mycelium often exudes acidic metabolites as it grows. Since a highly acidic environment can be harmful to the fungus and can encourage the growth of bacteria, the addition of chalk (calcium carbonate) to casing soil serves to maintain a slightly basic environment (a pH between 7.5-8.5). Gypsum (calcium sulfate) is added to help maintain a loose, airy structure, and to provide mineral nutrition to the growing fungus in the form of calcium and sulfur.
So-called “water crystals” are an optional ingredient you can add to your casing mixes. Made from a synthetic polymer chemically related to super glue, these crystals can absorb four hundred times their weight in water, and then release it slowly back into their surroundings. When fully hydrated, they look like clear gelatin.They are used in agriculture and gardening to conserve water usage and to protect plants from drying out completely between waterings. In a similar fashion, the addition of just a tiny amount of water crystals to a casing layer will serve to keep it hydrated and minimize the need for constant misting. A single flush of mushrooms can rob the casing and substrate of a huge amount of water, and these crystals can provide your cultures an extra level of protection from drying out.
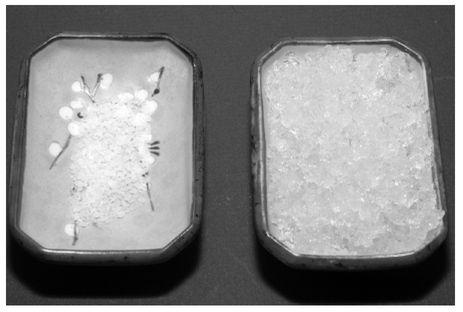
One half-teaspoon of “water crystals” before and after the addition of water.
Though they are a synthetic material, water crystals have been tested and shown to be non-toxic and environmentally benign. Over time they degrade com-pletely to carbon dioxide and water. They have even been scientifically tested to be safe when used in mushroom cultivation. Button mushrooms (Agaricus bisporus) grown in their presence were shown not to degrade or incorporate the chemical components of the gel.23 The crystals come in two varieties: ones made from sodium or others made from potassium. Since high levels of sodium are harmful to many fungi, be sure to get the kind made from potassium. Because the crystals degrade when heated, they must be added after sterilizing or pasteurizing the casing soil.
While some growers recommend sterilizing casing soils before use to minimize contaminations, we have found this step unnecessary as long as the components are kept clean and dry to begin with. However, if you want to be extra careful or you find that you do have trouble with contamination in your casing, a quick pasteurization may help. A simple method for pasteurizing small quantities utilizes a microwave oven. Just place the moist, prepared casing soil in a heatproof container or bag (large oven bags, the kind used to cook turkeys, or heavy plastic freezer bags are ideal) and microwave it on high for approximately 15 minutes. Make sure to leave the bag or container unsealed to prevent it from bursting. Allow the bag to sit for 10 minutes, and then microwave again for 15 minutes more. If you don’t have a microwave, you can also sterilize it in a pressure cooker for 45 minutes at 15 psi, or bake it in a 350˚ oven for 2 hours.Allow to cool completely before using.You may need to add more water to get the casing soil back to field capacity, since some moisture will inevitably be driven off upon heating.
We have provided three basic casing recipes, just to give you a sense of the variations utilized, and to take advantage of the materials you may have readily available.While all are equally effective for cultivating P. cubensis, we prefer the pure vermiculite formula for a number of reasons. For starters, it is exceedingly simple. Compared to other casing materials, coarse vermiculite is very easy to remove from the base of the harvested mushrooms. Vermiculite is created by a high-heat process, so it is very clean, almost completely resistant to contamination, and need not be pasteurized before use. Most importantly, since vermiculite is an inorganic material, it provides no nutrition to the fungus. As a result, overlay, a condition wherein the casing becomes overcolonized by mycelium and tightly bound together, rarely occurs with its use.
Note: Inhaled vermiculite dust is known to be very harmful for the lungs. For health and safety, a painter’s dust mask should be worn when first opening and working with it. Once the vermiculite is moistened, it ceases to release dust and is no longer dangerous.
Casing Soil Recipes
(All formulas are given on a by volume ratio.)
Pure Vermiculite
10 parts coarse vermiculite
1/2 part gypsum (Ca2SO4)
1/2 part chalk (CaCO3)
1/2 part gypsum (Ca2SO4)
1/2 part chalk (CaCO3)
Peat Moss Casing
10 parts peat moss
1/2 part gypsum (Ca2SO4)
1/2 part chalk (CaCO3)
1/2 part gypsum (Ca2SO4)
1/2 part chalk (CaCO3)
“50/50” Mix
5 parts peat moss
5 parts coarse vermiculite
½ part gypsum (Ca2SO4)
½ part chalk (CaCO3)
5 parts coarse vermiculite
½ part gypsum (Ca2SO4)
½ part chalk (CaCO3)
To each of these formulas, you may add ½-teaspoon water crystals per liter or quart of casing soil. Always add these after any optional heat treatment.
No matter which recipe you decide to use, the method of preparation is similar to that used in the PF Tek to bring the material to field capacity. Though colonized substrates and casing soils are less subject to contamination than earlier stages of growth, it is always a good idea to wear gloves and keep your workplace, tools, and containers as clean as possible.
1. Thoroughly mix all the ingredients together in a large, alcohol-sterilized container.
2. Reserve approximately 10 percent of this mixture.
3. Add water to the remainder until it is saturated, and you just begin to see free flowing water.
4. Add back the reserved dry mixture. If properly moistened, squeezing a handful of the soil should yield a few drops of water. Sterilize or pasteurize if desired, and allow to cool completely. Test a sample to make sure that it remains sufficiently moistened, and add water if needed.
5. Add water crystals (½ tsp per liter/quart), if desired.
6. Apply the casing to the substrate a little at a time, making the layer as even as possible. In order to maintain an open and airy structure, avoid packing it down. The thickness of the final layer should be from ½ inch to 1 inch.

Applying casing to a tub of grain. The right half of the container has not been cased yet.
After the casing layer has been applied, the container should be immediately placed under fruiting conditions, inside a bag or enclosure beneath lights. Mist it lightly and regularly using a hand sprayer filled with a 0.3% peroxide solution (1 part 3% H2O2 mixed with 9 parts water), set to create as fine a mist as possible. During the casing colonization phase, the water requirements of the fungus are minimal, and spraying once or twice a day should be sufficient to replace any loss due to evaporation.Take care not to water it too forcefully or heavily. It is better to water the casing layer lightly and often, rather than soaking it all at once, which can tend to mat it down.
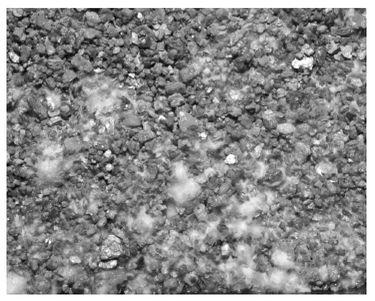
Mycelium emerging within the casing layer. The denser areas of growth will be filled in with small amounts of additional casing soil to allow even growth across the entire layer.
Soon the mycelium will begin to penetrate the casing layer, poking up through it in areas where it is thinner than others.With a clean spoon, apply additional amounts of moistened casing soil to patch these spots, so that colonization of the entire layer will proceed as evenly as possible.
Overlay
It is generally best if primordia form deep inside the casing layer rather than on top of it, to avoid the problem of overlay. Overlay occurs when vegetative (i.e., non-fruiting) mycelium is allowed to colonize the entire casing layer, which then becomes tightly bound together. An overlaid casing soil is impenetrable to water and gases, and the mycelium within it soon dies.The best way to avoid overlay is to initiate fruiting immediately after casing, and to make sure that the ideal conditions for fruiting are present from the start. Overlay is most likely to occur when the air is stuffy, very humid, and most of all, too warm.
Fruitbody initiation occurs when the mycelium in the casing senses a temperature and humidity difference between the substrate and the ambient atmosphere. As it grows from the substrate into the casing, it eventually reaches a boundary where moisture and temperature levels fall, signifying to the mycelium that the vegetative phase is over and it is time to begin fruiting. Very high humidity and warmth prevent the recognition of this boundary, so the mycelium just keeps right on growing, and because it has nowhere else to go, it just grows over itself, creating overlay.
By placing the freshly cased substrate under lights immediately, misting regularly, allowing adequate ventilation, and taking care not to overheat your growing area, the culture will fruit as soon as it is ready, and overlay will not occur.
Scratching
Once the casing layer is applied, it is best to avoid touching or manipulating it so you don’t damage the fragile primordia or introduce contaminants. However, despite your best efforts, there may still be occasions when you experience overlay. If so, you can rescue a casing layer by scratching it.
To scratch a casing layer, simply take a clean metal fork, sterilize it with rubbing alcohol, and gently scratch the casing down to the top of the substrate layer (try not to touch the casing with your hands as you do so). Loosen it up as much as possible, while maintaining an even depth. Mist the scratched casing lightly, and allow it to incubate in your fruiting chamber as before. If you are scratching more than one container, always sterilize your fork after each one to avoid spreading hidden contaminants.
The mycelium in older overlaid casings is potentially already dead, and carries a higher likelihood of contamination. Thus, this method is most effective when done as early as possible after the problem is discovered.
Contamination
While casing soils themselves are generally resistant to contamination, the mycelium itself is less so, particularly as it ages. Usually contamination sets in only after several flushes of mushrooms have been harvested and the substrate is nearly exhausted of nutrients. If contamination occurs early in the casing phase, it is probably an indication of a problem resident in the substrate or the casing soil itself, and the culture is best discarded in favor of starting with a clean one. As elsewhere, attempting to “save” a contaminated culture is usually not worth the frustration, and is only likely to spread the contamination to other containers.
There is one type of contamination unique to casing soils that you may occasionally encounter.This is Dactylium dendroides, otherwise known as “cobweb mold,” for its wispy, web-like appearance. It begins as small pinpoints of fine, white fuzz on top of the casing layer, and quickly grows to cover it completely. Cobweb mold is easily spread between containers at the slightest air disturbance, so contaminated cultures should be removed as soon as they are discovered. If allowed to proliferate, it will eventually attack and digest any mushrooms or primordia in the container, reducing them to a slimy mush.
Occasionally beginning growers will mistake the initial growth of mushroom mycelium into the casing layer for cobweb mold.True cobweb mold grows as a fuzzy layer on top of the casing soil, while the mushroom mycelium emerges through it from below. In addition, mushroom mycelium, while it may look wispy at first, will quickly thicken in appearance.
The occurrence of cobweb mold can be prevented by maintaining adequate air exchange inside the fruiting container, avoiding excessive humidity levels, and pasteurizing the casing soil prior to application.
11
FRUITING AND HARVESTING
Many other cultivated mushroom species require a drop in temperature or an increase in humidity to stimulate fruiting, but P. cubensis does not. Given a humid environment, sufficient gas exchange, and enough light, P. cubensis will fruit spontaneously, often before the mycelium has broken the surface of the casing layer. A number of growers recommend elaborate misting or fanning systems and cold-shocking the culture by chilling it overnight in a refrigerator in order to initiate fruiting, but we have found these methods unnecessary. So long as the strain being cultivated is a vigorous fruiter and has its basic conditions met, it should thrive. Therefore, your efforts are best spent on finding a robust fruiting strain early on, rather than working hard to fruit a weak one.
If you are experienced with the “PF Tek,” much of this chapter should already be familiar to you. In a few days to two weeks after casing, you should see primordia, tiny mushrooms in their most immature stage, beginning to form (see photos of this stage in the color section, page 74). Ideally, they will form inside the casing layer and will not be visible until they are already well-formed miniature mushrooms. Once they have achieved this size (around ½ cm), they tend to grow astoundingly fast, and can seem to reach full size almost overnight. As they grow, they will draw water from the underlying substrate and casing layer, so be sure to increase misting as needed (while always taking care not to over water.)
Harvesting
Once the mushroom has reached an appropriate size for efficient spore dispersal (usually somewhere between 3 inches and 6 inches in height), it ceases growing and its cap widens, opening to expose its gills to the atmosphere. The best time to harvest your mushrooms is just prior to this point, when the veil is stretched but not broken, since after this point the mushrooms will no longer gain any real weight. In addition, you don’t want spores falling onto your casing soil and containers. Given the astronomical numbers of spores produced by a single mushroom, this can make quite a mess; in addition, the gases released by germinating spores can potentially inhibit further fruiting.
The easiest way to tell when a mushroom is getting ready to open is to pay close attention to the partial veil, the thin protective membrane that covers the gills. Initially, the cap is completely inrolled (looking much like those old-fashioned globe-on-a-pole streetlamps), and the partial veil is hidden. As the cap starts to expand, the veil emerges as a circular, light-colored band around the bottom hemisphere of the cap. (Please see the visual reference for this stage in the color section, page 62.)
Once the cap begins to flatten out, the partial veil gets stretched beyond its ability to expand and begins to tear, pulling away from the outer edges of the gills. Eventually, the partial veil detaches from the cap entirely and its remnants remain attached to the stipe in a skirt-like ring, known as an annulus. (Again, see the color section, page 63.)
Ideally, you want to pick your mushrooms as soon as the partial veil is visible, or at the latest, by the time the veil begins to break.
Harvesting the mushrooms is as simple as grasping them at the base and twisting gently while pulling up and away from the casing. Any part of the mushroom that remains in the casing will rot, so take care to remove it all, down to the base of the stipe, using forceps or a pair of clean chopsticks if necessary. Try not to touch the casing layer directly with your hands, and take special care not to damage less-developed mushrooms or primordia nearby. Sometimes, it is impossible to avoid disturbing or uprooting nearby mushrooms when harvesting. In this case, it is better to remove these “babies” too, rather than leaving them behind. Disturbing them often severs their connection to the substrate and they stop growing and eventually rot. Don’t worry too much about the occasional loss, since whatever energy the culture would have expended on these fruits will be diverted to the next flush.
Usually, a fair amount of vermiculite will be stuck to the base of the harvested mushrooms, leaving behind a divot in the casing layer. Once you are done harvesting, simply fill these holes with fresh, properly moistened casing soil, mist thoroughly, and return the container to the fruiting area.
During the period immediately following a harvest, increase misting frequency and quantity significantly, in order to replace the substantial amount of water removed from the casing in the harvested mushrooms.
Each container should produce three to five crops, or flushes, of mushrooms with a week or so of recovery time between each flush. Generally the first few flushes are the most abundant. After the fourth or fifth flush, the substrate will be depleted, the mass of substrate will have visibly shrunk, and the number of mushrooms that form will be minimal. At this point, the containers should be discarded, since the mycelium in them will begin to die.Weak or dead mycelium is likely to become contaminated with molds, which could then spread to your healthy cultures.
Cleaning the Harvest
In the long run, it is easier and much better looking to clean off the mushroom stems when they are fresh rather than after they have been dried. Any casing soil remaining on the base of harvested mushrooms can be removed by gently scraping it off with a knife in a downward motion.
Yields and Biological Efficiency
Just how many mushrooms should you expect to harvest from a particular amount of substrate? To answer this question, we need to refer to the concept of Biological Efficiency, or B.E., a term created by the commercial mushroom industry. The biological efficiency of a mushroom is its inherent ability to convert substrate into mushrooms; A B.E. of 100% means either a 25% conversion of the wet mass of the substrate into fresh mushrooms, or a 10% conversion of the dry substrate into dry mushrooms. In other words, at 100% B.E., 100 g dry wheat berries could be expected to produce around 100 g fresh mushrooms, or 10 g dry. P. cubensis is a fairly robust species, and commonly achieves yields much higher than 100% B.E. (perhaps as much as 200%, or a 20 g per 100 g dry substrate). However, it is generally recommended that you not try extracting every last mushroom from your containers, and instead start fresh ones. Usually after the third or fourth flush the number of fruits produced will greatly decline and the culture will become susceptible to contamination, which could then easily spread to healthy cultures nearby.
12
AFTER THE HARVEST
Preserving Mushrooms
Fresh mushrooms can be stored in the refrigerator for up to a week without rotting or losing potency. They should be placed in a breathable container, such as a paper (not plastic) bag, or a sealable plastic container lined with a paper towel, with the lid slightly ajar. If you want to store your mushrooms for longer periods, you will have to preserve them somehow, since psilocybin and its related compounds rapidly oxidize and become inactive when exposed to the atmosphere.The simplest and most effective method of preservation is drying. Kept from light, heat, and moisture, dried psilocybin mushrooms can retain their potency for many months, even years.
The fresh mushrooms should be slowly dried under gentle heat (110˚ F or below) until “cracker” hard, and no longer spongy. Next, place them in a sealed container such as a zippered freezer bag or, better yet, a heat-sealed food storage bag. As much air as possible should be expelled from the bag before sealing it. For added protection, individual bags should be placed into a secondary sealed container before freezing. If space is at a premium, the mushrooms can be powdered in a spice mill or coffee grinder after drying, but they will not retain their potency as long as when kept whole, since more of their chemical components will be exposed to the atmosphere.
A kitchen food dehydrator makes an excellent tool for drying mushrooms, especially one that has a precise temperature control and a circulating fan.The best models circulate warm air in a horizontal direction, which results in even drying across all shelves. A makeshift food dehydrator can also be easily fashioned by constructing a wooden box with removable, sliding wire screen shelves and a 150-watt incandescent light bulb at its base as a heat source. Alternatively, mushrooms can be dried by placing them overnight in a warm oven or on a rack above a radiator. Whatever drying method you use, make sure to use gentle heat, 110˚F (43˚ C) or below. Mushrooms dried at higher temperatures will be bitter tasting and considerably less potent.

Freshly harvested mushrooms being loaded into a food dehydrator.
Spore Printing
Spore printing, like tissue cloning, is a method of preserving the genetic makeup of your cultures. Spores are the product of sexual reproduction, which means that a spore print will contain many different genotypes. As in human reproduction, each individual spore (or “child”) will be made from some random combination of characters from each of its two parent nuclei. Strictly speaking, a spore print will never contain exactly the same genetics as the mushroom it came from (unlike tissue cloning, which will). Nevertheless, Psilocybe mushroom species are typically quite stable from one generation to the next, and the great majority of spores in a print will behave almost identically to their parents. Spore prints can remain viable for years if they are kept from light, heat, and moisture. Thus, they represent a form of insurance in case a cloned strain loses vigor or is lost altogether.
Making a Spore Print
1. To take a spore print, you will need a mushroom on which the cap is flat and the gills are fully opened. (This is the one instance when you should let the mushroom develop past the globe-on-a-stick stage and allow the partial veil to begin to break before harvesting.) As with cloning, pick only the largest, most robust specimens for spore printing, and make multiple prints whenever possible.
2. With a clean, sharp knife, cut the stem of the mushroom just below where it attaches to the cap, so that when placed face down, it will be raised a millimeter or two above the printing surface. Make the cut as clean and flat as possible in order to provide a horizontal and stable base. With the tip of the knife, remove any traces of the partial veil from the gills. Avoid touching the gills directly.
3. Prints can be made on paper, glass microscope slides, or aluminum foil. Of these, glass or foil are best, because they can be sterilized by wiping them down with alcohol and drying before use, and their smooth surface texture allows the spores to be easily removed with an inoculation loop later on. Sterile plastic Petri dishes work nicely as well, provided the cap is small enough to fit comfortably within them. Take several prints on a single sheet of foil, leaving ample space between each cap, so that the foil can be folded over the print for stor age. If using slides, you will probably need to use several to contain an entire cap.
4. Sterilize the printing surface with alcohol and allow to dry.
5. Place the cap face down on the printing material, and cover it with an inverted container to maintain a humid environment and minimize air currents.
6. Within a few hours, the cap should have deposited its print onto the foil. Slower producing specimens can be left overnight to create a denser print, if necessary.
7. The completed print should be sealed to minimize contamination. If using foil or paper, cut out the print, and fold clean foil over it, taking care not to pressAbove: Taking spore prints on aluminum foil.Below: After several hours, the spore prints are complete.directly on the spores below. Seal the edges by folding them over. If you use glass microscope slides, cover the print with a sterile blank slide, and tape the edges.Labeled spore prints ready for storage.
8. Place the prints inside zippered storage bags, and mark them with the date and any other important information.
Spore prints should be stored in a spot that is away from light, moisture, and heat, but should not be refrigerated or frozen. Well-preserved spore prints can remain viable for many years.
13
OUTDOOR CULTIVATION
Outdoor mushroom cultivation offers a number of important advantages over growing indoors. Once an outdoor garden bed has been established, it will fruit annually for several years, until the substrate has been fully consumed. By periodically adding fresh wood chips to the mixture, or by creating a new bed nearby and using some of the original substrate as inoculum, the life of a bed can be extended almost indefinitely. Such beds require virtually no maintenance beyond keeping them moist during the drier, hotter months of the summer. Since wood chips are cheap, and can even sometimes be had for free, outdoor cultivation is inexpensive. Wood-based substrates are far less prone to colonization by bacteria and molds, so they can be handled openly, without fear of contamination. Finally, since they can be easily incorporated into just about any shady, out-of-the-way garden location, outdoor mushroom gardens are far more discreet and low profile than any other mushroom cultivation method.
Though the substrate colonization and fruiting stages of outdoor cultivation require very little maintenance, the early stages are more or less identical to those required for P. cubensis cultivation. Spores are germinated using the cardboard disc method, and the resulting mycelium is grown out on agar in Petri dishes, and then transferred to sterile grain. Once the grain spawn is fully colonized, it is used to inoculate small quantities of sterilized wood-based substrate.This wood-based spawn is utilized to inoculate a large quantity of wood chips, which are then employed to create the final bed. Once the fruiting substrate is fully colonized, fruitings commence when the ambient temperatures fall into the 40˚ F range, from mid-October until early winter in northern North America. Flushes appear once every two weeks or so, as long as temperatures remain constant.
Temperature Requirements
P. azurescens and the other lignicolous (wood-loving) Psilocybes will fruit outdoors only if your local temperatures drop into the forties each autumn, and stay there for several weeks or more. If you live in Florida or southern California, you will unfortunately need to stick to growing P. cubensis, or think about moving somewhere less balmy.
About Wood Substrates
The mycelium of the “wood-loving” species will grow readily on just about anything derived from trees, so long as it is either made from hardwoods, or derived from softwoods that have been stripped of their aromatic constituents, like most paper products. When it comes to fruiting, they are more particular, and will only do so from a mixture of hardwood chips and sawdust. Nevertheless, you have a wide variety of substrate options when it comes to the pre-fruiting stages of cultivation.Wood-based substrates are naturally resistant to attack by molds and bacteria, so they need not be sterilized before use. Substrates that are readily consumed by some fungi, while resistant to attack by other organisms, are known as selective for those species.
Wood is primarily composed of cellulose and lignin. Lignin molecules are long, cross-linked chains of phenolic organic compounds. The bonds that make up lignin are extremely chemically stable, which gives wood its characteristic hardness and longevity. As a log burning in your fireplace will confirm, there is a great deal of energy contained in wood, but it is bound tightly within the lignin latticework and is not easily accessed by most organisms. In fact, the only organisms that can break down and consume lignin are certain species of fungi. The fungi that possess the necessary enzymes to do so are called “lignicolous” or “wood-inhabiting” fungi, a group that includes the caramel-cap Psilocybes. The selectivity of wood substrates for lignicolous fungi is what makes them considerably easier to handle than materials like grain.
One interesting feature of these species is that cultures collected from the wild or from healthy cultivated beds are extremely resilient, capable of flourishing under circumstances that “virgin” pure spawn cultures would not survive. This is because exposure to various challenges stimulates an organism to express its full capabilities. Virgin cultures that have grown only on sterile media made of simple components have very few of their enzyme-producing genes activated. Free-living organisms, on the other hand, have had to compete daily with a wide range of other species, and have by necessity developed the capacity to survive in their presence. Such “acclimated” cultures gathered from the wild are surprisingly robust. They will grow readily on unsterilized substrates, and often seem to do even better on “dirty” substrates than on clean ones.
Of course, if you are starting from a spore print, this fact will be of little use to you, at least at first. You still have to use sterile methods in the early stages of the process: from cardboard discs to agar, then to grain, and finally to sterilized wood. But if you are fortunate to have access to an already established bed, either because you live where these mushrooms grow in the wild, or because you or someone you know has already gone to the trouble of creating one, you can forgo sterile methods altogether, and simply transfer mycelium to fresh substrate to create a new bed. At the end of this chapter, we have included several methods for putting such “naturalized” spawn to use.
Spore Germination (see chapter 7)
Spores should be germinated using the cardboard disc method or by streaking on non-peroxidated agar. After germination, the cultures should then be transferred to peroxide-containing agar for further use.
Cloning (see chapter 7)
Alternatively, if (wild-collected or cultivated) fresh mushrooms are available, they can be cloned onto agar and grown out. Besides its obvious convenience, cloning also guarantees the isolation of a reliable fruiting strain.
Another use of fresh mushrooms, a kind of “quick-and-dirty” cloning method, involves sandwiching the bottom part of their stems between layers of moistened corrugated cardboard. Over several weeks, the mycelium will grow out over the cardboard, which can then be used to inoculate fresh wood chips. This type of cloning is a quick way to generate spawn, since it avoids the need for sterile techniques altogether. A full description of the method is given at the end of the chapter.The photo section shows mycelium growing onto cardboard on page 76.
Peroxide and the Caramel Caps
We have experimented with Psilocybe azurescens, P. cyanescens, P. cyanofibrillosa, P. subaeruginosa, and P. bohemica, and all of them grew vigorously in the presence of peroxide, whether on agar or grain.
Agar Culturing/Strain Selection
Unless you live where these mushrooms grow wild, or have access to someone else’s mushroom beds, you will have to start from spores, so your cultures will initially be composed of numerous different strains. The woodloving Psilocybe species cannot be easily forced to fruit indoors, and, unlike P. cubensis, they do not exhibit much in the way of useful mycelial characteristics. (On agar, they tend to grow smooth, silky mycelium. Once transferred to wood-based substrates, however, they do produce beautiful, strongly forking rhizomorphs. See color photos of P. azurescens agar culture and rhizomorphs on page 76.)
There is no real shortcut method for isolating a vigorously fruiting strain from these species. In order to insure that one does not potentially propagate a non-fruiting strain, it is important to perform as few transfers as possible before moving the culture to a grain- or wood-based matrix. With each transfer, the number of strains in any given culture diminishes greatly.To keep these numbers relatively high, cultures should be placed on agar soon after the spores have germinated, and then immediately moved to grain. Grain cultures are easily shaken to redistribute their genetic contents, maintaining diversity.
“PF Tek” for Woodlovers
Alternatively, if you have spore-water syringes rather than spore prints (or you make your own), you can use a PF-style technique to create multi-strain cultures of the woodloving species. Using the techniques described in Chapter 6, you can produce PF-style half-pint jars of mycelium on brown rice cakes, which can then be used to directly inoculate a wood-based substrate. Because each jar will contain many different strains, and because several intervening steps are eliminated, this method conserves the total number of strains in the final substrate.
Once fully colonized, the sterile PF cakes should be tipped from their jars directly into bags of sterilized wood chips that have been prepared as described for use in grain-to-wood transfers. One jar is used per bag. After the bags have been heat sealed, the cakes can be broken up by gently crushing them from the outside of the bag. Finally, the bags should be shaken to fully distribute the mycelial fragments.
Grain Spawn
Jars of grain spawn are prepared and inoculated exactly as described in chapter 8.
Biology of Rhizomorphs
You will recall that many strains of P. cubensis display rhizomorphic growth, with their mycelium organized in dense, root-like bundles of hyphae. (P. cubensis rhizomorphs are pictured in the color section on page 77.)
Most of the time, the presence of these structures is a sign of a strong fruiting strain, and if you look closely you might note that most primordia form along their length and tips. Rhizomorphs are like the veins and arteries of the fungal body, moving and distributing nutrients, minerals, water, and waste products throughout the system, while the finer, single-celled hyphae are the equivalent of capillaries. Primordia form along rhizomorphs precisely because that is where the fungus can most rapidly shuttle the large quantities of nutrients needed for mushroom formation.
The wood-loving Psilocybes rarely form distinct rhizomorphs on sterile substrates, although they do so after being introduced to a natural setting. Unlike the relatively simple and fragile rhizomorphs of P. cubensis, these species create thick, rope-like structures, with an inner core of bundled hyphae contained within a hard, protective outer layer.The outer shell serves to prevent attack from other organisms and to minimize the loss of water under drying conditions, while the core shuttles water, nutrients, and oxygen wherever they are most needed throughout the wider network. These rhizomorphs are most pronounced around the base of developing mushrooms; they are so thick and resilient that they will pull free of the substrate along with the picked fruit, still clinging tenaciously to pieces of wood chips (see color section page 77).
Rhizomorphs also perform a “reconnaissance” function, reaching out far in advance of the expanding colony in search of new material to consume. One of the great advantages of this approach is that every rhizomorph comprises a large number of individual hyphal strands, each of which can individually branch out to surround and penetrate a portion of the new substrate material. Once a new source of food is found, the rhizomorphs then signal to the mother colony to follow in its wake, presumably by releasing a specific chemical messenger.
Wood-Based Primary (Sterilized) Spawn
Once a sufficient number of grain jars have been prepared, they should be used to inoculate bags or jars of wood, rather than larger quantities of grain. Because of the partial selectivity of wood-based substrates, the sooner you make the leap from grain to wood, the less likely it will be that your cultures succumb to contamination.
In this chapter, we make a distinction between three types of wood-based substrates. (Keep in mind that these terms are our own, coined only to delineate the different stages of this particular process; you are unlikely to find them used quite this way in any other cultivation manual.) Primary spawn is sterilized wood spawn that is inoculated from grain cultures in relatively small quantities. Secondary spawn refers to spawn derived from primary spawn, and any subsequent expansions thereafter. Unlike primary spawn, secondary spawn need not be made up of sterile materials.The fruiting substrate is simply the final generation of material prior to fruiting, and is often identical in composition to secondary spawn.
Wood Chips
Whether used as spawn or final fruiting substrate, the choice of tree species for your source of wood chips and sawdust is a critical one. While we have heard of success using wood from firs or other conifers, we find them less than ideal for good growth. All of the wood-loving species described in this chapter grow best on wood from deciduous tree species, whether soft woods such as alder and poplar, or hardwoods like oak or maple.

Hardwood sawdust pellets, birch dowels, and alder chips.
Ideally, you will have access to a wood chipper and can make your own wood chips from wood harvested locally. Small- to medium-sized branches or small saplings are ideal (no need to chop down whole trees), and are best when harvested in the late winter or early spring, before the foliage has emerged and when sugar content is highest. Wood harvested at other times of year is usable, but all leafy parts must be carefully removed before chipping to avoid rotting. If you cannot chip your own, you might be able to obtain fresh wood mulch from your local highway or parks department at low cost, or even for free. However, it can be difficult to control what tree species it contains, so it is important to specify ahead of time if possible that you need chips from hardwood trees, or better yet, from one particular species.
If a local source of wood chips is unavailable, there are a number of mail order and online sources for hardwood chips, sold for use in barbecues and wood smokers. Because of the relatively large quantities of chips needed to make a full-sized (4’ x 4’ x 8” or greater) bed, shipping costs can be high, but discounts for bulk orders can often be negotiated to offset some portion of the cost. (Hardwood chips for barbecuing can sometimes be found available locally in kitchen, hardware, or grilling supply stores as well.)
As for which tree species to use, we have found both alder and oak to work well. Since alder is a relatively soft hardwood, it is much more quickly colonized by the fungus, and is best for the rapid establishment of a new bed. Denser woods like oak are colonized more slowly, and beds made of them will last much longer before needing to be replenished. Ideally, you could either combine light and dense woods for your bed, or add the denser wood to the bed after the softer wood has been fully colonized.
Other suitable broadleaf species include maple, eucalyptus, birch, cottonwood, poplar, elm, walnut, beech, hickory, dogwood, aspen, yew, and ash. Other hardwoods will work as well, but you might want to experiment with small quantities before using untested species to create large beds. It is safe to assume that if the fungus seems to grow happily on the wood, it should fruit on it as well.
Freshly cut wood chips should be sufficiently moist for use and require only a brief soaking under a hose to prepare. Dried wood chips should be soaked in room temperature water for 12-48 hours to moisten them. Once they are fully hydrated, the chips will sink to the bottom of the container. If you only need small quantities, chips can also be simmered in a pot of water on the stove for about an hour, or until they sink. Drain thoroughly before using.
Spiral-Grooved Dowels
When making primary spawn, the choice of tree species is less than critical, so you can also use spiral-grooved dowels. Such dowels are readily available from woodworking suppliers as furniture-joining pegs; the best ones are 1 to 2 inches long and ¼-inch or  -inch in diameter. They are usually made from birch, and will be designated as such. They are most commonly used in mushroom cultivation on logs, where the colonized pegs are pounded into holes around the circumference of the log.The spiral groove around the outside of the peg provides a maximal surface area from which the mycelium can leap off onto subsequent substrates. Birch dowels are readily colonized by lignicolous Psilocybes and are a very effective material for use as primary spawn.
-inch in diameter. They are usually made from birch, and will be designated as such. They are most commonly used in mushroom cultivation on logs, where the colonized pegs are pounded into holes around the circumference of the log.The spiral groove around the outside of the peg provides a maximal surface area from which the mycelium can leap off onto subsequent substrates. Birch dowels are readily colonized by lignicolous Psilocybes and are a very effective material for use as primary spawn.
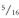 -inch in diameter. They are usually made from birch, and will be designated as such. They are most commonly used in mushroom cultivation on logs, where the colonized pegs are pounded into holes around the circumference of the log.The spiral groove around the outside of the peg provides a maximal surface area from which the mycelium can leap off onto subsequent substrates. Birch dowels are readily colonized by lignicolous Psilocybes and are a very effective material for use as primary spawn.
-inch in diameter. They are usually made from birch, and will be designated as such. They are most commonly used in mushroom cultivation on logs, where the colonized pegs are pounded into holes around the circumference of the log.The spiral groove around the outside of the peg provides a maximal surface area from which the mycelium can leap off onto subsequent substrates. Birch dowels are readily colonized by lignicolous Psilocybes and are a very effective material for use as primary spawn.Sawdust
To insure rapid colonization, good drainage and air exchange, and longevity, it is important that the substrate matrix be composed of a variety of particle sizes, particularly when working with larger amounts of material in the latter stages of the process. Substrates made up of only fine materials (less ¼-inch in diameter) are quickly colonized, but can become water-logged or overly bound up, while those comprising only large wood chips can be slow to colonize and will have a tendency to dry out quickly. Therefore it is useful to add finer materials such as sawdust to your substrate mixture when making secondary spawn and the final fruiting substrate. (Primary spawn should be made from wood chips or dowels alone because it is generated in small, sealed containers and in limited quantities. Making primary spawn from larger, harder materials confers optimum resistance from contamination.)
Wood chips made with a chipper/shredder usually contain a good mix of particle sizes, while commercially available chips tend to be more uniform and will require the addition of finer materials. One good source of sawdust is hardwood pellet fuel, especially since the high heat used in their manufacturing renders the pellets more or less sterile. A brief soak in warm water is all that is needed to restore the pellets into sawdust form.
Sawdust has a finer particle size and greater surface area than wood chips, so it is somewhat more vulnerable to attack by wood-loving contaminants. Therefore, when using sawdust in non-sterile (secondary) spawn, it is best to choose harder woods like oak rather than alder to offset this susceptibility.
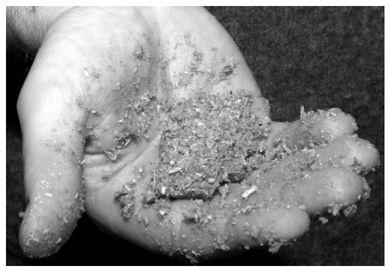
Properly moistened sawdust will hold its shape when formed into a ball.
The exact amount of water needed to moisten your sawdust depends on the species of tree and the brand of pellets used, so you will need to experiment at first to avoid over-hydrating it. Properly moistened sawdust should yield a few drops of water when squeezed into a ball, and hold its shape when released.
Timing
Because the mycelium of the woodloving Psilocybes grows best when ambient temperatures are between 40˚ and 75˚ F, the best time to plant a bed is in the early spring, after the threat of frost has fully passed. This will give the fungus several months to colonize the bed before the heat of summer sets in and it enters dormancy. Then, in late summer and early fall, it will have a month or two to revive before the fruiting season commences.
You should plan experiments so you have a sufficient quantity of spawn to plant in the spring. It takes 2-4 months to go from spore print to secondary spawn (1 to 2 weeks each to germinate spores, grow out on agar, and colonize grain jars, a month to grow out primary spawn, and 1 to 2 months to produce secondary spawn), so you should plan to start the process in January to be ready for a spring planting.
If this is not possible, then the second best opportunity to plant a bed is in late summer, when the hottest months have passed. Depending on the degree of colonization of your material and the length of time before winter arrives, beds established in late summer or early fall may or may not fruit during the first year.
In our experience, beds planted during the summer rarely prosper. As soon as temperatures climb above 75˚F, the fungus refuses to grow. Any uncolonized substrate in the bed becomes vulnerable to contamination by molds, many of which thrive in summer heat. More than once have we tried starting a bed in the summer, only to find it a pile of brown mush by season’s end.
Note that primary spawn remains viable at low temperatures, which means you can at least begin the process at any time of the year. A bag or jar of primary spawn can be stored for up to a year under refrigeration, and then used to create secondary spawn immediately prior to planting in springtime.
Spawn Rates
Spawn rate is the ratio of spawn to fresh substrate.The higher the spawn rate, the faster and more successful the colonization will be. When growing outdoors, you are working with unsterilized substrates and potential contaminants are everywhere, so using high spawn rates is particularly important to insure success. This is why we recommend that you aim for a rate of 20% or greater (i.e., a 4:1 ratio of fresh substrate to spawn) when growing the woodloving Psilocybe species.
Wood-Based Primary Spawn
When making the initial leap from grain-based to wood-based substrates, it is always best to sterilize the spawn before inoculation. Even though wood is resistant to contamination, sterilizing it at this stage insures that the fungus will gain a solid foothold on the substrate before it is used further. Once the primary spawn is fully colonized, it can then be freely used to inoculate unsterilized wood products.
Be sure that the grain spawn you use in this stage is fully colonized and absolutely free of contamination. Because wood-based cultures do not contain peroxide, it is especially important that you eliminate any possible avenues of contamination from your inoculum.
Wood-Based Primary Spawn
For photos of primary spawn, see p. 78.
For one standard spawn bag or 6 quart jars:
3 lbs. dry wood chips (or 4 lbs. fresh)
or
3 lbs. birch dowels
For one standard spawn bag or 6 quart jars:
3 lbs. dry wood chips (or 4 lbs. fresh)
or
3 lbs. birch dowels
1. Soak or simmer wood chips or dowels until properly hydrated. Drain thoroughly.
2. Load into bags or jars.Wood chips usually have somewhat sharp edges, so take care whenever handling bags that they do not puncture.
3. Load into your pressure cooker and pressure cook for 1.5 hours at 15 psi.
4. When cooled fully, inoculate with grain spawn at a rate of around ½ 1 cup per bag or ¼ cup per quart jar.
5. Seal containers and incubate at room temperature.
6. Within a few days to a week, you should see mycelium beginning to leap off the grain onto the wood.
7. Shake the containers once a week or so, taking care not to puncture the wood chip bags as you do.
8. Once the containers are fully colonized, they can be used to inoculate larger quantities of material or refrigerated for later use.
Contamination
Though wood-based substrates are highly resistant to contamination, numerous unwelcome fungi do grow on wood and can pose a problem for growers. Such contaminants rarely occur on properly prepared and sterilized substrates, but they do occasionally arise on untreated materials.
One of most commonly encountered contaminants on wood substrates is Trichoderma viride. This fungus is a fast-reproducing mold that produces powdery, green-blue spores. A color photo of Trichoderma viride is on page 79.
In addition to consuming wood, it is a pathogen on other fungi, parasitizing both mycelium and fruitbodies, and eventually killing the host outright. If your wood-based cultures are contaminated with Trichoderma, it is best to carefully discard them.
Occasionally, woody cultures will turn soft, moist, and darken in color. This is probably the result of contamination by one of the many “brown-rot” fungi (see photo, page 79). This type of contamination is most commonly encountered in outdoor beds, in portions of the substrate that have yet to be colonized by the mushroom. It can be avoided by using a sufficiently high spawn rate employing the layering method we describe below, and giving the fungus a sufficient amount of time to colonize its bed before the heat of summer arrives.
Secondary (Non-Sterilized) Spawn
Once the primary spawn is fully colonized, it can then be used to create a larger quantity of material. A 5-pound bag of spawn could be used to inoculate a full bed of wood chips, but would result in a fairly low spawn rate (10% or less), and the bed would be prone to contamination by unwanted fungi. By first multiplying the amount of pure spawn five- or tenfold, then using a 25% spawn rate, the final colonization will proceed rapidly and the health of the bed will be maximized.

A bag of
P. azurescens
primary spawn ready for use.
Large plastic opaque tubs or storage bins are ideal containers for use at this stage. They should be washed thoroughly with soap and water, and wiped down with alcohol before use.
The inoculated substrate should be covered with a piece of clean, wet corrugated cardboard that is held down with a brick or similar heavy weight. The cardboard helps to maintain high moisture levels during colonization, while the weight serves to compress the substrate, which helps to promote fast colonization.
It is best to use sawdust derived from harder woods such as oak in unsterilized secondary spawn, rather than softer species like alder, which are susceptible to contamination when ground into finer particles.
As always, make sure to wash your hands thoroughly and/or wear clean gloves before making secondary spawn.
Secondary Spawn
For photos of this process, see pp. 80-81.
Per container:
5 lbs. dried wood chips (or 10 lbs. fresh)
2 lbs. sawdust or fuel pellets, preferably from a harder species such as oak
For photos of this process, see pp. 80-81.
Per container:
5 lbs. dried wood chips (or 10 lbs. fresh)
2 lbs. sawdust or fuel pellets, preferably from a harder species such as oak
1. Moisten wood chips and sawdust as before, and mix together.
2. Line the bottom of your clean container with a piece of clean corrugated cardboard to absorb any excess moisture.
3. Break the contents of the spawn bag into small chunks by crushing it between your hands (from the outside of the bag).
4. Load a quarter of the new material into the container.
5. Inoculate each container with a portion of the spawn, aiming for a final rate of ¼ to 1 bag per container (higher rates will produce faster colonization times.) Mix the spawn thoroughly throughout the new material.
6. Repeat steps 4 & 5 until all material and spawn have been used.
7. Cover the substrate with a piece of moistened, corrugated cardboard cut to fit, and lay a few bricks over it to hold it down.
8. Incubate containers at room temperature.
9. Mist the cardboard with a spray bottle periodically to keep it thoroughly moistened.Inspect the substrate once a week to check for growth.
10. After several weeks, when the container is approximately one-third colonized, you can remove the cardboard, and, wearing a clean pair of gloves, gently stir the contents by hand. Replace the cover and incubate as before.
11. The substrate should be fully colonized within 1-2 months.
Secondary spawn produced in this manner can be stored in a cool place for a month or so, but it does not keep as well as sterilized spawn. At this point, it is best to use it as soon as possible to create an outdoor bed.
Chances are that the cardboard covering should itself be colonized by the mycelium as well. Save it, since it can be separately used to create more spawn, as described on p. 152.
Location
Ideally, you want to situate the bed beneath a large plant, such as a rhododendron bush or an ornamental tree, which will serve to maintain moisture levels and protect it from direct sunlight. In addition, because the fruiting substrate is made up of wood chips, such beds are perfectly camouflaged, appearing more or less like any other mulched garden area. On the other hand, when they are fruiting they do become considerably more conspicuous, so it is always best to situate the bed in a private, out-of-the way spot.
Alternatively, if you do not have access to a suitable private location of your own, beds can also be established on public land, though this is a somewhat trickier proposition, since the chosen spot must be both well concealed and self-maintaining. You wouldn’t want to place it somewhere where unsuspecting, curious strangers might be tempted to pick the mushrooms that will appear each year. The location should be hidden well enough to be invisible to the uninformed. Possibilities include concealing it beneath the branches of an overhanging tree or bush. It is also unlikely that you will be able to drag a hose or watering can out to the bed whenever it needs watering (and even if you could, that would surely arouse suspicion), so it is necessary that the spot receive regular and appropriate amounts of moisture. Therefore, landscaped grounds maintained by automatic sprinkler systems, such as those in parks or around large municipal or private office buildings, are ideal.

A
Psilocybe azurescens
bed. A layer of grass helps to conceal the presence of the fungus during the off-season and to promote healthy fruitings.
Containers and Substrate Depth
While beds can simply be placed in a wide, shallow hole, they tend to do best if somewhat contained. For this reason, we like to line our beds with bricks, rocks, or wooden boards (painted with a soy-based waterproof paint or sealer). When placed within a well-defined space, the fungus seems to sense when the edges of the substrate have been reached. At this point, it stops searching for new sources of food and prepares itself to fruit. Whatever the final outer dimensions of your bed, you should aim for a substrate depth of 8-10 inches.
Fruiting Substrate
The final fruiting substrate is prepared similarly to secondary spawn, using 20-40 pounds dried wood chips (or 30-60 pounds fresh) and 10-15 pounds sawdust for each 10- to 20-pound container of secondary spawn prepared. A 4’ x 4’ x 10” bed will require approximately 40 pounds of substrate. In order to maintain a sufficiently high spawn rate and insure rapid colonization, it is best to make the bed smaller if the initial amount of spawn is limited.You can always expand the size of the bed by adding more substrate in following years.
The one important difference between this procedure and the one used to create secondary spawn is that the spawn is placed in one contiguous layer over the fresh material, rather than mixed throughout. Since this stage takes place in the great outdoors where there are a host of other fungi around that could also consume the fresh wood in the bed,“capping” the new material with a full layer of spawn protects it from such attacks.24
Creating the Bed
1. Moisten the wood chips and sawdust as in previous methods.
2. Line the bottom of the bed with clean cardboard to lift the substrate up off of the soil below. Poke a few holes in it to insure adequate drainage.
3. Fill the bed with all of the fresh substrate.
4. Gently break apart the container of secondary spawn and lay it over the bed.
5. Cover the bed with one or two layers of moistened corrugated cardboard, and secure it at each corner with bricks or other heavy weights.
Incubation
Water the bed as often as necessary. As long as the cardboard covering is adequately moistened, the bed below should be fine. Water with a hose sprayer, being as gentle as possible so as not to disturb the developing mycelial mat. Pay particular attention to water levels during the hottest months of the summer, when beds are most susceptible to drying out. In late September or early October, when ambient temperatures drop below 65˚F, remove the cardboard covering completely and add a casing layer as described below.
Casing & Companion Planting
Because wood substrates do not retain moisture particularly well, the top inch or so of beds have a tendency to dry out once the cardboard covering is removed in the fall. Such drying out causes the mycelium in the upper layers of the bed to die back. Since fruiting occurs at only the very top surface of the mycelial mass, it is particularly important to avoid this effect. We found that adding a casing layer to the top of the bed helps to maintain high moisture levels at the fruiting surface and protect the fragile primordia as they develop. It also provides a healthy matrix on which a variety of beneficial microorganisms will grow, further stimulating fruiting.
The casing layer should be ½- to 1-inch deep and should consist of moist, pure sphagnum peat moss. One of the advantages of using peat moss is that it has a built in humidity indicator: when moist, it takes on a dark, chocolate brown color and has a distinct orange-brown cast when dry.You should mist the casing layer lightly whenever necessary in order to maintain high humidity.
Optionally, you can throw a handful or two of perennial grass seed over the casing layer and water them in. The wood-loving Psilocybes, and P. azurescens in particular, fruit commonly from wood chips buried beneath grasses (see photo, p. 81). The grass helps to maintain high moisture levels at the fragile fruiting surface.There is also some evidence that it may provide additional nutrients to the mycelium, stimulating fruitbody development.
Fruiting
Fruiting should commence once temperatures drop to around 45˚ F for three or four days in a row, and will continue until frost sets in. As long as environmental conditions remain conducive, flushes should occur once every two weeks or so. Aside from harvesting the mushrooms as they mature, all you need to do at this point is make sure that the bed remains well watered.
Winter Dormancy
Once winter arrives, the beds cease fruiting, and enter a period of dormancy. All of the species described in this chapter are tolerant of periods of below-freezing temperatures, and can survive even the harsh winters of many northern climates. If you live in an area that receives long periods of below-freezing temperatures, you will need to take special measures.To insure the survival of an outdoor garden, cover your beds with a layer of straw, leaves, plastic, cardboard, or fabric shade cloth, to insulate and maintain adequate moisture levels in the bed throughout the winter months.

Psilocybe subaeruginosa
fruiting beneath a rhododendron bush.
Alternatively, if local winter temperatures are extremely harsh, you can dig up the bulk of the bed and store it indoors in covered trays or tubs in a cool dark place until warmer temperatures return.

A
P. azurescens
box ready for winter hibernation. A layer of dried leaves and several sheets of burlap shade cloth help to insulate it from the cold.
Restoring Depleted Beds
Once established, woodchip beds should fruit for two or three years without further amendment. However, to insure the longevity of a bed, it is best to provide additional material for the fungus to consume each year.
In spring, after the bed has had a month or so to recover from the winter freezes, dig down with your hand into its core in several locations. If the woodchips are still quite firm, and the bed is uniformly colonized by healthy white mycelium, it can be amended by simply stirring more fresh, moist woodchips throughout the mixture. Scrape away most of the old casing layer, add the fresh chips, and incubate as before.
If, on the other hand, the bed is old or otherwise depleted, it is best to use healthy portions of it to create a new bed, either in the same location or elsewhere.
Outdoor (“Naturalized”) Spawn Transfers
If you want to establish a new bed from an old one or from one found in the wild, all you need do is collect healthy mycelium and substrate and mix it with fresh substrate. Carefully dig around in the bed to extract whatever healthy colonies of mycelium remain, avoiding any portions that are rotten, brown, or dead. Thoroughly consumed substrate, which is in all likelihood no longer viable, will be dry, brittle, and light, and should crumble easily on handling. Healthy woodchips should be firm and moist, and enclosed in a sheath of mycelium.
Assuming you recover a sufficient quantity of material to inoculate the desired amount of fresh substrate (still aiming for a 4:1 or better spawn ratio), you can simply use this to prepare a new bed. If you have less than you ideally need, you should expand it further before building the final substrate, as described above in the preparation of secondary spawn.
Stem-Butt and Cardboard Spawn
Another effective method for creating new spawn from another established bed utilizes fresh mushrooms rather than substrate to create the next generation. Stem butts (the lower portion of the stipe and any attached substrate) are simply placed between layers of moist corrugated cardboard and incubated in an enclosed container. The mycelium in the stem will soon revert to a vegetative state and begin to colonize the cardboard. In time, the individual islands of mycelium from each stem butt will unite, and the cardboard will be covered in a thin layer of mycelium.
Keep in mind that the stem butt method does require a fair number of fresh mushrooms (5-10 or more) to produce useable spawn in a reasonable amount of time. If all you happen to have is one or two speci-mens, you are better off cloning them to agar and using sterile techniques to produce the desired amount of substrate.
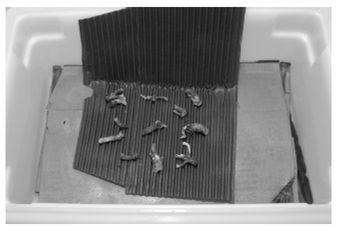
P. azurescens
stem butts placed on moist cardboard for cloning.

The same stem butts begin to colonize the cardboard one week later.
This is also a practical way to make further use of the cardboard insulation removed from incubating substrates; by the time the underlying material is colonized, the cardboard should be covered with mycelium as well. Instead of throwing this material out when removing it from the substrate, it can be transferred to fresh material and reused.

P. azurescens
rhizomorphs begin to leap off onto the cardboard.
To use a myceliated cardboard sheet, remove one of its thin outer layers to expose the corrugated core within, and place it on top of or beneath a fresh container of moistened wood chips. If you have more than one sheet, you can alternate them between layers of chips, lasagna-style. Once the chips are fully colonized, they can then be used as spawn to create larger quantities of substrate.

A sheet of cardboard removed from the top of a tray of colonized wood chips.
Can I Grow Woodlovers Indoors?
Given how potent these species are and how limited annual yields from an outdoor bed are relative to P. cubensis, it is tempting to grow the “caramel caps” year-round indoors under more controlled settings.You might wonder if you can just grow them out in trays or tubs of woodchips and then force them to fruit by chilling them to 40˚ F for a few days in a refrigerator. The answer to this question is maybe: folks have gotten woodloving Psilocybes to fruit indoors, but yields have generally been quite low compared to similar amounts of substrate planted outdoors.
It appears that temperature is not the only important influence on fruitbody formation for these species. As is the case with a number of other cultivated mushroom species, the woodloving Psilocybes seem to require a biologically active substrate to fruit properly, if at all. Something about the presence of other microorganisms, probably bacteria, in the substrate tells the fungus to fruit. A curious aspect of these species is that they only seem to display strong rhizomorphic growth (a precursor to fruitbody formation) after being exposed to “raw,” unsterilized substrates, perhaps in response to the presence of other organisms. In all of the indoor success stories we have seen documented, fruiting occurred only after very long incubation periods exposed to the external atmosphere, by which time the substrate would have accumulated quantities of microorganisms, particularly at the fruiting surface.
Clearly there is much more to learn about how these species behave, and we strongly encourage readers to experiment further. Perhaps you will be the one to break the code that tells how to coax the woodloving Psilocybes to easily fruit indoors. If you do, please share your results with the rest of us!
14
THE CHEMISTRY OF PSILOCYBE MUSHROOMS
The chemical compounds found in Psilocybe mushrooms are a complex of related tryptamines, substances comprising an indole ring and an amine connected by a two-carbon chain. Don’t worry too much if you find the actual chemistry in this chapter a little esoteric. The important take-away lesson is this: these compounds are all closely related to the common amino acid L-tryptophan, from which the fungus constructs them, and to serotonin (5-hydroxytryptamine), a major mammalian neurotransmitter, which helps to explain their human pharmacological effects.
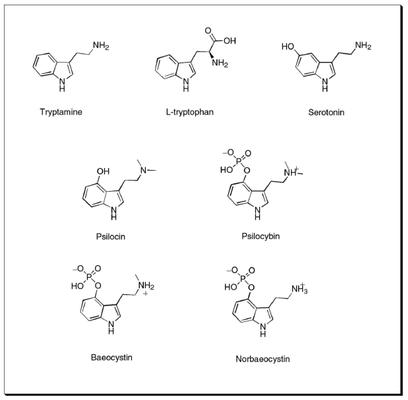
Psilocin and its phosphate ester psilocybin are the most common compounds found in these fungi, while baeocystin and norbaeocystin are generally present only in trace amounts, if at all. Psilocybin is rapidly converted in the body to psilocin via the removal of its phosphate group, making the two compounds more or less identical in effects and potency after their different molecular weights are taken into account. The psychoactive effects of baeocystin and norbaeocystin in isolation are not well understood, but there is some evidence to suggest they modulate the psychoactive effects of psilocin and psilocybin. Their presence in varying amounts may help to explain the common anecdotal reports of subjective differences from one species of Psilocybe mushroom to another.
No doubt owing to the myriad legal obstacles to public scientific study of these mushroom species, almost nothing concrete is known about why these chemicals are present in these mushrooms in the first place. Nature rarely does anything without a practical purpose, especially when the action is energetically costly, as it surely is here. These compounds must serve some evolutionary advantage for the survival of these species; otherwise, the resources that go into synthesizing them would be better spent on making greater numbers of spores or larger fruitbodies, for example. One might be tempted to argue that these molecules ensure the survival of the mushrooms species since their presence encourages human beings to grow and propagate them for their psychoactive effects. However, such logic gives human beings far more credit than they are due, since these fungi got along just fine for millions of years without our assistance. The chemicals they contain must have had some important purpose in their day-to-day struggle to compete and survive in the natural environment.
Many organisms produce chemicals that have some positive or negative ecological effect on other organisms in their environment. Such compounds are often called “secondary” metabolites, since they are not known to have any primary effect on the internal functioning of the organism itself, but they are better thought of as allelochemicals, compounds whose effect is meant to influence other organisms. Allelochemicals can serve three interrelated functions: semiotic, competitive, and/or symbiotic. Semiotic compounds signal to the recipient to come closer or to stay away. The powerful scents of many flowers are meant to attract pollinating insects or animals, while the bitter compounds in the leaves of other plants discourage foraging by other organisms. Competitive chemicals can be defensive, offensive, or both at once. The sting of a honeybee does not do any lasting harm to its victim, but it teaches an important lesson in avoidance all the same.That of the hornet or wasp, on the other hand, is primarily intended to kill its insect prey. Symbiotic allelochemicals confer mutual benefit to both producer and recipient: the hummingbird receives nourishing nectar in exchange for (unwittingly) spreading pollen from one flower to the next.
What allelochemical function psilocybin might serve for the fungi that produce it is not known, but most likely it is a defensive one. It may help prevent other organisms from competing for resources or from feeding on the tender and nutrient-rich hyphae as they explore the environment of the substrate. Perhaps these chemicals kill or inhibit the growth of snails, slugs, or worms.25 It is also possible that they have antibiotic properties, helping to keep bacteria or other fungi from attacking the fungus.The fact that they are produced in much greater concentrations in the fruitbodies than in the naked mycelium lends support to the idea that they serve a defensive function: if the goal of the mushroom life cycle is to produce and release as many spores as possible, it is the fruits that require the greatest protection from attack.
Even if these molecules are not synthesized by the fungus for the “purpose” of encouraging an ongoing relationship with human beings, there is no question that they do have profound effects on the human brain. It could be argued that these effects are accidental, but not coincidental: human beings evolved in the same environment as worms, bacteria, and fungi, and are made up from the same basic chemical and biological building blocks. Many organisms have tryptamine-like molecules in them; though they are closely related chemically, the functions they serve are often as diverse as the organisms themselves. Biologists like to use the lock-and-key metaphor to describe the activity of chemicals on biological systems: when the key (the chemical) is inserted into the lock (the receptors on or inside the cells of the organism), some effect occurs. Because all organisms evolved from a common ancestor, the number of such chemical “keys” is limited, while their effects are not.What happens when you put psilocybin into a slug or into a human depends upon the location and the function of the receptors with which it interacts.
Exactly how psilocybin produces the effects that it does on the human brain is still very much a mystery, both because of the profound complexity of the organ26 and the legal restrictions placed on the study of psychedelic molecules. Nevertheless, it is believed that its primary effect is mainly the result of its interaction with certain serotonin receptors. Neurobiologists refer to two generic types of active molecules: agonists and antagonists.An agonist binds to a receptor with a similar effect as the actual neurotransmitter, while an antagonist blocks the effect of the neurotransmitter. Returning to our lock-and-key metaphor, an agonist is a key that fits and turns the lock, though perhaps with more or less efficiency than the neurotransmitter itself, while an antagonist merely sticks in the lock, preventing the real key from getting in. Psilocybin and related molecules are thought to be serotonin agonists. They bind to receptors and act upon them much like serotonin does, but with a slightly different affinity. While the effect at each individual receptor site might be subtle, their overall effect on the human mind is unquestionably profound.
Psilocybin Safety
Given their powerful human psychological effects and their theoretical functions as defensive allelochemicals, one might reasonably wonder whether the compounds found in Psilocybe mushrooms might be in any way toxic to human health. In fact, there is no evidence to suggest that they are at all poisonous. First of all, they are unlikely to be toxic, given that they have such a long history of human use without a single attributed death. In addition, these molecules have been subjected many times to traditional toxicology tests, which showed them to be quite innocuous. Psilocybin has an LD-50 (or 50% lethal dose) of approximately 280mg/kg in rats and mice, which means that you need to give the test animals 280 milligrams of psilocybin for every kilogram of body weight in order to kill half of them. Roughly speaking, what this means for humans is that the average 80-kilogram adult male would need to ingest 22 grams of pure psilocybin, or something like 500 grams of dried Psilocybe cubensis mushrooms, in order to earn a 50% chance of dying! By comparison, caffeine, widely considered to be a benign human drug, has an LD-50 in rats of 192mg/kg, making it some 1.5 times as “toxic” as psilocybin.
15
THE PSILOCYBE MUSHROOM EXPERIENCE
We assume that you would not have gone to all the trouble to learn to grow psilocybin-containing mushrooms without some previous direct experience with their psychoactive effects, and some understanding on how to use them. If you are not familiar with their effects, we commend your enthusiasm and aplomb for having come this far on the mere promise of the delights and wonders that these mushrooms can reveal. We assume that before you cast off onto these vast, still mostly uncharted, and always mysterious waters, you will have done your homework. Consult with others who have gone before you, either in person, online, or in print.27 The more you know before you set off, the better prepared you will be for the mysteries you will encounter, and the more treasures you will be able to carry with you on your return.
We have a few recommendations for how to make the most of the mushroom experience. Of course, you should take such advice with a grain of salt since, as always, your mileage may vary.
Fresh vs. Dry
Since it is highly likely that your cultivation projects will provide far more mushrooms than you could possibly need at any one time, you will probably be drying them for long-term storage and later use. Psilocybe mushrooms can be ingested fresh, but there are two factors to consider if you choose to do so. First, fresh mushrooms are approximately 90% water by weight, so you will need to multiply the weight of your dosage by a factor of 10 when using fresh mushrooms.
In addition, many people (your humble authors included) find fresh mushrooms considerably less digestible than dried ones for some unknown reason. More than once have we eaten freshly picked mushrooms to find ourselves afflicted with cramps, indigestion, and general discomfort for much of the voyage. Drying them seems to eliminate whatever factor produces these effects. One way to avoid indigestion when using fresh mushrooms is to make an infusion from them (as described below) and discard the solids after steeping.
If you do choose to eat fresh specimens, make sure they are clean, firm, and recently picked. Older, soft fruits can harbor bacteria and should be discarded.
Dosage
Recommending a dosage regime for mushroom ingestion is complicated by the great variability in the potency of mushrooms, both among different species, and between strains or flushes of the same species. In addition to the great variation in potency among different mushrooms, there is also a very real and often wide variation in individual sensitivity to psilocybin. What might be a threshold dose (in other words, the lowest possible amount needed to feel any effect whatsoever) for one person may be a whopper for another. It is quite important that each person be well acquainted with his or her own sensitivity before experimenting with an unfamiliar sample or dosage. When in doubt, it is always best to err on the side of caution; you can always take more next time around, or even later on, after the effects of the first dose have made themselves fully felt. (Ninety minutes is usually sufficiently long to wait before taking a booster dose.)
One important influence on individual sensitivity is body mass; because the drug is distributed more or less uniformly throughout the body after ingestion, a heavier person will require a larger dose to achieve the same effect as someone of small stature. For that reason, recommended dosages of pure compounds are given as milligrams per kilogram of body mass (mg per kg). One kilogram is equivalent to 2.2 pounds.
Recommended Dosages by Species
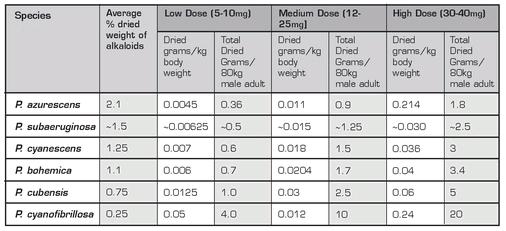
Once you understand how to use this chart, you should easily be able to figure out an amount in grams for each species at any one of three dose levels.
The first column lists all of the species we describe in this book, ranked in order of potency, from highest to lowest. The second column gives percent weight of all alkaloids present in each species; for example, P. azurescens contains a maximum of 2.1% alkaloids, or 21 milligrams of alkaloids per dried gram. The numbers in this chart are based on all the literature studies we examined. (No such studies exist for P. subaeruginosa; all numbers here are estimates based upon ample anecdotal evidence suggesting it is a moderate to highly potent species.) The remaining columns provide recommended amounts across three different doses, both in dried grams per kilogram of body weight, and an “average” dose, for reference purposes.
One example should be sufficient to make this clear. Say you are a slim woman, weighing in at a mere 118 lb, and require a medium dose of P. azurescens:
118 lb ÷ 2.2 lb/kg = 53.6 kg x 0.011 g/kg = 0.59 grams
Therefore a 118-pound woman would ingest 0.59 grams of dried P. azurescens mushrooms in order to get a medium dose—between 12-25 mg of alkaloids. Note that 0.59 grams is a significantly lower amount than the 0.9g “average” dose appropriate for an 80 kg / 176 lb burly adult male.
Dosage Levels
Low Dose: 5-10 mg alkaloids
At this level, the mushrooms have just begun to make themselves felt, producing a gentle, amorphous altered state, not unlike an “up” marijuana high. The body feels energized and the mind alert. Senses and perceptions are heightened. Colors may seem brighter and more vivid, music and sounds often seem to be more distinct and crisp, tastes are enhanced, and so on. While perceptions of the external world are lightly altered, actual audio or visual hallucinations are unlikely to occur at this dosage. Such low doses are amenable to use in public settings, such as art museums or musical concerts, since one’s outward appearance will be normal enough to avoid attracting unwanted attention from strangers.28 This is also an excellent dose level for daytime exploration and contemplation of the natural world.
The effects at this level generally commence within 30 minutes, and last from 2-4 hours.
Medium Dose: 12-25 mg alkaloids
At this level, both closed- and open-eyed visual hallucinations can arise. Initially, and at the lower range of the medium dosage, these are mainly highly colored, vivid geometric patterns, not unlike elaborate, living Oriental rugs. Synesthesias, where two or more senses cross or overlap are not uncommon at this level. Tastes, smells, touch, music and other sounds can enhance and synergize with the visuals in astonishing and surprising ways, and vice versa. At slightly higher doses, abstract visions can give way to more pictorial images, both familiar and strange.
With sufficient experience and comfort with these dosage levels, one can venture out into the natural world, to great effect, but we recommend avoiding contact with strangers whenever possible.
The effects at this level generally commence within 30 minutes, and last from 3-5 hours.
High Dose: 30-40 mg alkaloids
At these doses, the sky really is the limit.Terence McKenna referred to such amounts as “heroic doses,” since each experience is inevitably a voyage into uncharted waters. What you will find we cannot say, since the experience will be highly personal and always singular. We do however recommend that you make sure to find yourself in comfortable and protected settings before you begin, free from distractions and unwanted surprises. Silent darkness, alone or with a guide is ideal for such voyages. Don’t even think about venturing out into the “real world” at this dose level. You probably won’t be able to stand up, much less walk with any amount of coordination anyway.
At high doses, the mushroom experience generally commence within 30 minutes, and lasts from 5-7 hours.
Higher Doses
We do not recommend doses much beyond 0.5mg/kg, even for the most experienced and intrepid traveler. After this point the law of diminishing returns sets in, and the experience becomes longer and more intense without necessarily being more rewarding.
As we explained in chapter 14, psilocybe alkaloids are extremely benign to human health and it is practically speaking impossible to take an “overdose,” at least one that is physically harmful to human health. If you find you have taken more than you should have, accidentally or otherwise, rest assured that, despite the intensity of the experience, you will surely survive. Even at extremely high doses, the experience will last no longer than 8 hours, with the most intense part over far sooner than that.
Monoamine Oxidase (MAO) Inhibitors and Psilocybe Alkaloids
If you are currently taking monoamine oxidase medications of any kind, you should not ingest psilocybin (or any psychedelics, for that matter.) These drugs are designed to deactivate the human enzyme system responsible for the metabolism of many drugs and common food toxins. With MAO inactive, compounds the body would otherwise degrade can have unpredictable and potentially dangerous effects. If you are taking MAO inhibitors for depression (their most common indication), you will need to wait until you have stopped taking them before experimenting with psilocybin.
Another psychedelic, the Amazonian brew ayahuasca, deliberately combines an MAO inhibitor from the vine Bannisteriopsis caapi with a tryptamine-containing plant to produce its effects. Some clever “psychonauts” have used this model to create a “mycohuasca” by combining Psilocybe mushrooms with B. caapi or other MAO-inhibiting plants, dramatically potentiating and altering their effects. We don’t recommend doing so, but if you decide to experiment with such a thing yourself, please be careful and, as always, do your homework.
Tolerance
When psilocybin is used more than once a week, tolerance generally occurs.The exact causes behind this phenomenon are not well understood, but the overall effect is that the brain becomes temporarily desensitized to a specific drug after each exposure. While tolerance to psilocybin can be overcome by significantly increasing the dose, it is best to simply wait at least a week between voyages to give the brain (as well as your psyche) an opportunity to return to baseline.
Methods of Ingestion
Most people simply chew and swallow the dried mushrooms. For those who find them somewhat less than palatable, it is simple enough to make an extract, since the alkaloids in Psilocybe mushrooms are freely soluble in both ethanol and hot water.
No matter what method of ingestion you use, it is advisable to fast for a least 6 hours before using Psilocybe mushrooms, to minimize indigestion and to maximize absorption of the alkaloids.
Mushroom Tea
Simply make a pot of your favorite herbal tea using one and one-half cups of water per person, preferably using aromatic herbs and spices such as mint, cinnamon, or cloves to help mask the taste of the mushrooms and to calm the stomach. After steeping the tea for 10 minutes or so, pour it into a second pot containing the requisite amount of fresh or dried mushrooms. Cover this and allow to steep for at least one hour, stirring occasionally. Strain and pour into the appropriate number of teacups. The remaining mushroom solids may be eaten, but this is generally not necessary, since most of the alkaloids will have infused into the tea.
High temperatures will rapidly degrade alkaloids, so the steeping liquid should never be allowed to boil once the mushrooms have been added. If the tea is not used immediately, it should be refrigerated. Once prepared, mushroom tea should be used within 48 hours.
Alcohol Extract
For a longer lasting preparation, consider making an extract. Crushed or powdered mushrooms can be soaked in high-proof alcohol (150-proof or greater) such as rum or Everclear, using 25-50 milliliters of alcohol per dose. After soaking for 3 days or longer, the extract can be filtered or decanted and stored for several months or longer without considerable loss of potency.
16
CONCLUSION: WHERE TO GO FROM HERE
If you made it to this point in the book, chances are you are eager for more. We know of few people who have gotten a taste of the wonders of mushrooms through the cultivation of Psilocybes and simply left it at that. We have met more than a few mycologists or mushroom cultivators who were initially drawn in with the simple goal of growing a few mushrooms for themselves, only to discover that the experience was just the first step on the path to a lifelong passion for mycology, or even a full-fledged career in the field. There are at least two reasons for this phenomenon. First, as you are now surely aware, cultivating mushrooms is not trivial work, even with the many improvements that have been developed over the years.The skills and discipline that must be practiced in order to succeed at it are too precious to be simply abandoned once a particular goal has been met. Once acquired, such talents naturally demand continued application. Second, the science and behavior of fungi are simply so marvelous and fascinating that it is nearly impossible to avoid being drawn deeper into their world. Like a mycelial colony expanding exponentially through a rich substrate, the mind awakened to the wonders of mycology must continue to explore toward its farthest reaches.
Should you find that this book has awakened your curiosity, we have offered recommendations for further reading in the resources section of the appendix. Psilocybe mushrooms and other gilled Basidiomycetes are but the tip of the iceberg. We suggest exploring the world of edible and medicinal mushroom cultivation next, since it offers many delights. There are a number of edible mushroom species to which the skills described in this book are easily adapted. The common and tasty oyster mushroom, Pleurotus ostreatus, is perhaps the ideal beginner’s edible, since it is even easier to grow than Psilocybe cubensis. It fruits quickly and abundantly from nearly any substrate you can throw at it: paper, wood, straw, grains, spent coffee grounds, just about anything containing a moderate amount of cellulose. In addition, its mycelium is so fast growing that it easily outruns competing molds and bacteria, making it one of the most contamination-resistant fungi around. Finally, it is easily cloned onto agar from grocery store-collected specimens.29

Oyster mushrooms fruiting from toilet paper rolls inoculated with grain spawn.
When it comes to using grain as a fruiting substrate, Psilocybe cubensis is the exception rather than the rule. While Pleurotus ostreatus will fruit from grain, it does much better on woody substrates. Other species are more demanding in their nutritional requirements, and they should be thoroughly researched before attempting to grow each of them. Other indoor species for the beginning edible mushroom cultivator to try are Pleurotus pulmonarious (the “Phoenix Oyster”), Hypsizygus ulmarius (the “Elm Oyster”), and Agrocybe aegarita (the “Poplar Mushroom”).
A number of edibles can be grown outdoors using almost exactly the same methods utilized to grow the wood-loving Psilocybes. One of our favorite outdoor-cultivated edibles is Stropharia rugosoannulata (or “Wine-Cap Stropharia,” photo on p. 82). Its fruits are meaty, firm, and delicious and are often produced in great quantities. Much like Psilocybe azurescens, the wine-cap can be grown on a bed of wood chips. It fruits in summer and early fall rather than during the colder months, and sometimes does not fruit until the second full season after planting. Fruits can often be enormous, occasionally weighing as much as a pound each, but they are more flavorful and better textured when picked at the earlier “button” stage, before the cap has expanded and the partial veil has broken. A number of other delectable edible species conform to more or less the same cultivation strategy; additional suggestions include Hypholoma capnoides (the “Smokey Gilled Woodlover”) and Hypholoma sublateritum (the “Brick Cap” or “Cinnamon Cap,” photo on p. 82).

Wine-cap stropharia fruiting from a bed of alder chips cased with peat moss.
Some fungi are “psychedelic” without ever being ingested by humans. Although they are inedible, bioluminescent fungi, species that produce their own light from mycelium and fruitbodies, are a delight to grow and observe. There are at least forty well-documented bioluminescent Basidiomycetes, but perhaps the easiest to grow and most radiant species is Panellus stipticus. Its mycelium and diminutive, oyster-like fruits shed an eerie, warm green light that can be easily seen with the naked eye when viewed in total darkness. Exactly why Panellus and similar fungi luminesce remains a mystery to science, though one theory is that they use light to attract night-flying insects to assist them in spore dispersal.
From tasty edibles to spooky glow-in-the-dark mycelium, the world of mycology offers a nearly endless supply of delights and wonders. We hope that you have enjoyed learning more about these beautiful, fascinating, and truly extraordinary organisms here, and that the Psilocybe mushrooms you grow will impart to you valuable insights about yourself and the world. Wherever your mushroom pursuits lead you next, we wish you safe travels and the best of luck.

A jar of
Panellus stipticus
mycelium growing (and glowing) on grain.
Appendix A
Quick Reference for Substrate and Casing Recipes
PF Technique Jars
Per ½ pint jar:
40 mL (scant ¼ cup) organic brown rice flour
140 ml (½ cup) vermiculite, plus extra for casing layer
40 mL (scant ¼ cup) organic brown rice flour
140 ml (½ cup) vermiculite, plus extra for casing layer
For full instructions, see p. 85.
Malt Yeast Agar (MYA) Medium
22 g agar
12 g light malt extract
1 g yeast extract
¼ tsp organic grain flour (rotate among oats, cornmeal, amaranth, rice,
millet, rye or any other starch or sugar you can think of)
5 g hardwood sawdust or wood fuel pellets
1 L tap water
8mL 3% hydrogen peroxide (optional, added after sterilization & cooling)
12 g light malt extract
1 g yeast extract
¼ tsp organic grain flour (rotate among oats, cornmeal, amaranth, rice,
millet, rye or any other starch or sugar you can think of)
5 g hardwood sawdust or wood fuel pellets
1 L tap water
8mL 3% hydrogen peroxide (optional, added after sterilization & cooling)
For full instructions, see p. 95.
“Anything” Agar Medium
20 g anything
22 g agar
1 L tap water
8 mL 3% H2O2 (optional, added after sterilization & cooling)
22 g agar
1 L tap water
8 mL 3% H2O2 (optional, added after sterilization & cooling)
For full instructions, see p. 97.
Grain Spawn
For additional information, see p. 110.
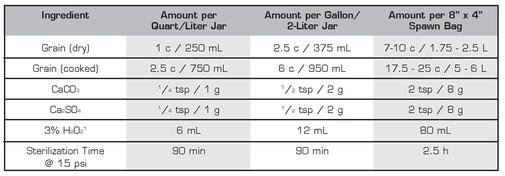
Casing Soils
For more information, see p. 124.
All formulas are given on a by volume ratio.
Pure Vermiculite
10 parts coarse vermiculite
½ part gypsum (Ca2SO4)
½ part chalk (CaCO3)
½ part gypsum (Ca2SO4)
½ part chalk (CaCO3)
Peat Moss Casing
10 parts peat moss
½ part gypsum (Ca2SO4)
½ part chalk (CaCO3)
½ part gypsum (Ca2SO4)
½ part chalk (CaCO3)
“50/50” Mix
5 parts peat moss
5 parts coarse vermiculite
½ part gypsum (Ca2SO4)
½ part chalk (CaCO3)
5 parts coarse vermiculite
½ part gypsum (Ca2SO4)
½ part chalk (CaCO3)
To each of these formulas, you may add ½-teaspoon water crystals per liter or quart of casing soil. Always add these after any optional heat treatment.
Wood-Based Primary Spawn
For more information, see p. 145.
For one standard spawn bag or 6 quart jars:
3 lbs. dry wood chips
or
4 lbs. fresh wood chips
or
3 lbs. birch dowels
3 lbs. dry wood chips
or
4 lbs. fresh wood chips
or
3 lbs. birch dowels
Wood-Based Secondary Spawn
For more information, see p. 147.
Per tub:
5 lbs. dried wood chips (or 10 lbs. fresh)
2 lbs. sawdust or fuel pellets, preferably from a harder species such as oak
5 lbs. dried wood chips (or 10 lbs. fresh)
2 lbs. sawdust or fuel pellets, preferably from a harder species such as oak
Wood-based Fruiting Substrate
For more information, see p. 149.
Per 4’ x 4’ x 10” bed:
20-40 pounds dried wood chips (or 30-60 pounds fresh)
10-15 pounds sawdust each 10- to 20-pound container of secondary
spawn prepared
20-40 pounds dried wood chips (or 30-60 pounds fresh)
10-15 pounds sawdust each 10- to 20-pound container of secondary
spawn prepared
Appendix B
GLOVE BOX & FLOW HOOD PLANS
While hydrogen peroxide will protect your agar and grain cultures from contamination, there are times when it cannot be used, such as when germinating spores on cardboard discs or agar, or transferring cultures onto wood or paper pellets for storage. In these cases, it is necessary to physically, rather than chemically, eliminate contaminants from your cultures and your work area. There are two basic approaches growers use to maintain sterility in the absence of peroxide: a glove box or a laminar flow hood.
CONSTRUCTING A GLOVE BOX
A glove box is essentially a “room within a room,” an enclosed work space large enough to hold your tools and materials, but small enough to easily maintain a relatively sterile interior atmosphere. The inside of the glove box is rendered more or less sterile by wiping down its insides with alcohol and misting the air inside with a dilute bleach solution before use.True glove boxes are completely sealed off from the external environment, and their interiors are accessed by having the operator slide her hands into attached gloves. In our simplified design, the operator simply slides her hands into the box through holes on two of its sides. By keeping hand movements inside the box to a minimum, the chances of contaminants falling into the culture containers remains extremely low. A glove box is a cheap and effective tool for maintaining sterility in any home lab setting.
All you really need to create a glove box is a container large enough to create a suitable workspace, preferably made of materials that are easy to modify and chemically resistant enough to stand wiping down with alcohol before each use. We chose to utilize a cardboard box in our system, since it is both light and collapsible for storage when not in use.
Materials
A clean cardboard box, 2’ x 2’ x 2” (or thereabouts)
1 roll of glossy, white contact paper
1 clear, vinyl shower curtain (or similar sheet of thick completely translu-
cent plastic)
Sticky-backed Velcro tape (10’ or more)
Clear packing tape
1 roll of glossy, white contact paper
1 clear, vinyl shower curtain (or similar sheet of thick completely translu-
cent plastic)
Sticky-backed Velcro tape (10’ or more)
Clear packing tape
1. With a sharp pair of scissors or a utility knife, remove the four top flaps from the box.
2. Cut out a wedge from the box by drawing a line from two corners of the box that are diagonal from one another, meeting at a point halfway down the edge of the corner in between them.The short side you cre ate will be the front of the box, pointing toward you as you work.
3. Line the entire inside surface of the box with contact paper, including the upper faces of all four bottom flaps.
4. Cut two 6-inch circles out of the two adjoining front sides of the box, 3 inches from the bottom and 6 inches back from the front corner.
5. Lay the clear plastic sheeting over the box, and cut it to fit the large opening, leaving at least 3 inches of overlap on all four sides.
6. From the remaining plastic, cut two 8” x 12” squares.
7. Attach the cover to the box by taping it to one of the long rear sides.
8. Use the Velcro tape to attach the plastic top to the box along the other three sides. Be sure to use one continuous strip of tape along each side to create a continuous seal.
9. Attach the squares of vinyl with tape to the box so they hang loosely over each of the armholes. Place a strip of Velcro tape along the bottom edge to secure them when not in use.
10. Use Velcro tape to attach the outer bottom box flaps to the inner ones. The various Velcro attachment points allow the box to be easily flattened when not in use.
Using Your Glove Box
1. Wipe all inside surfaces including the vinyl cover and armhole flaps with an alcohol-soaked paper towel.
2. Close the armhole flaps.
3. Load your prepared materials and tools into the box.The items you are working with should, of course, be more or less sterile at this point.
4. Holding the cover off loosely with one hand, liberally mist the insides of the box with a 10% bleach/water solution31. You should mist it enough that the air within is fully saturated, but not so much that you make a mess of your materials.
5. Seal the top of the box completely, and allow it to sit for 5 minutes or so.
6. Wash your arms and hands thoroughly with soap and water, don your gloves, and wipe down your gloves and forearms with alcohol. (Make sure the alcohol has fully evaporated before you go near any open flames.)
7. Lift the flaps and let them drape over your hands and arms as you access the inside of the box.
8. Work carefully and deliberately, putting your hands only as far into the box as necessary, and avoiding any fast movements.
9. When you have finished your work, carefully seal all containers before opening the cover of the glove box to remove them.
10. A note of caution: If you are using an alcohol lamp inside your glove box, be careful to keep it as far back in the box as possible, to prevent the heat of the flame from melting the vinyl cover.
Laminar Flow Hood
While glove boxes can be useful tools to help minimize contamination in cultures without peroxide, they are not 100% effective, and are limited by their small size. They are perfect for inoculating a single sleeve of Petri dishes or a few grain jars, but become unwieldy and lose efficiency as soon as you try working with larger quantities of materials. A much more effective and versatile tool for these purposes is the laminar flow hood. Composed of a fan that blows a continuous stream of air through a HEPA filter, the flow hood creates a wide sterile zone within which to work.
A high quality commercial flow hood can cost upwards of $500, but you can build one yourself for around half this much using our plans (or even less if you can locate a cheap surplus fan). The two most expensive items are a blower fan and the filter itself.
HEPA filters are available from mail-order and online air filtration supply houses (at the time of this writing, prices range from $80-100). Be sure to purchase one rated at 0.3 microns and 99.99% efficiency. They are available in both wood- or metal-framed versions. Either will do; metal-framed models are lighter and somewhat more expensive, but not really worth the additional cost.
In order to produce an adequate airflow across a 24” x 12” x 5.8” filter, you will need a fan that is rated between 450 and 500 CFM (cubic feet per minute). A number of online greenhouse and gardening suppliers sell 465CFM fans perfectly suited for this application for around $100 apiece, but similar fans can often be had more cheaply from auction sites or surplus parts dealers, so it is worth shopping around.
Keep in mind that once assembled this hood weighs about fifty pounds and takes up quite a bit of room. It is not exactly the kind of thing you will want to be lugging great distances whenever needed, so make sure you have enough room to store it somewhere relatively close to your work area.
Materials
Squirrel cage fan (450-500CFM)
24” x 12” x 5 ⅞” HEPA filter
A small box of 1 ½” wood screws (150 pieces)
4’ x 4’ sheet of ¾” plywood or particle board
8’ of 1” x 1” furring strip, cut into 2 12” and 2 24” lengths
8’ of 1” edge trim, cut into two 25 ¼” and two 13 ¼” lengths
Tube of all-purpose silicone sealer
2 3” or 4” steel handles
24” x 12” x 5 ⅞” HEPA filter
A small box of 1 ½” wood screws (150 pieces)
4’ x 4’ sheet of ¾” plywood or particle board
8’ of 1” x 1” furring strip, cut into 2 12” and 2 24” lengths
8’ of 1” edge trim, cut into two 25 ¼” and two 13 ¼” lengths
Tube of all-purpose silicone sealer
2 3” or 4” steel handles
1. Cut all the pieces for the box itself from the plywood sheet. Cut a hole in the top panel slightly smaller than the width of the blower outlet, 2 inches away from one of the long sides and centered across it.
2. Assemble the sides and top and bottom pieces first. It’s a good idea to drill pilot holes for the screws to prevent the wood from splitting. Leave the screws somewhat loose at this point.
3. Slide the back panel into the end of the box (the side closest to the blower hole in the top panel) and mount it flush with the other four sides.
4. Tighten all screws snugly.
5. Mount the four furring strip pieces around the inside of the box, 6 inches from the front face, creating a flange against which the filter will sit tightly.
6. Run a bead of silicone sealant along every seam on the inside of the box, and smooth it out with your finger to get a clean seal.
7. To mount the blower over the hole in the top panel, first hold the fan over the hole to determine where the pilot holes should go, and drill them. Run a bead of silicone around the edges of the holes, mount the fan, and screw it in place tightly.
8. Run a bead of silicone around the outward face of the flange, and then slide the filter up against it snugly. It might be a little tight going in. It should sit flush with the front edges of the box.
9. Cut the trim pieces to fit around the front of the box and hold the filter in place. Pre-drill pilot holes and screw them into the box (make sure to screw it into the outer edges of the box, not into the filter itself).
10. Paint the box if you want, let it dry, and then mount a handle on each end.

Using a Laminar Flow Hood
Always run the hood for 30 minutes or so before using, to blow off any particulates that might have accumulated on the filter since it was last used.
Remember that sterility is always highest close to the filter, and drops off as the airflow falls away. Therefore, you should always aim to keep the cleanest items closest to the filter, and “dirty” things further downstream. For example, when performing transfers from one Petri dish to another, the clean, sterile plate should be placed nearer to the filter than the colonized one, so that any particles present on outer surfaces of the older plate are blown away from the work area and not across it. Similarly, you should strive at all times to keep your hands and arms downstream of all sterile materials.
Appendix C
RESOURCES
This book covers several methods for cultivating a select number of Psilocybe mushroom species. There is a whole wide world of mycology beyond its pages that you will inevitably be drawn to explore once you have had a taste for its many wonders.The books, magazines, and Web sites listed here should provide you with a few good places to start your forays. They are all resources we consult frequently, and they come with our highest recommendations.
Field Guides
Arora, David. 1991. All That the Rain Promises and More. Ten Speed Press.
Arora, David. 1986. Mushrooms Demystified. 2nd Ed. Ten Speed Press.
Barron, George. 1999. Mushrooms of Northeast North America. Lone Pine Publishing.
Bessette, Arleen R., Alan E. Bessette, and William J. Neill. 2001. Mushrooms of Northeastern North America. Syracuse University Press.
Lincoff, Gary. 1991. National Audubon Society Field Guide to North American Mushrooms. Alfred A. Knopf.
Since no single resource is ever definitive, you should always consult as many as possible when attempting to make a field identification of an unknown mushroom. The above five titles are among the best field guides to North American fungi in print. While the Arora books are specific to Western states, and the Bessette and Barron titles cover the Northeast, you should find all of them useful, no matter where you live.
Stamets, Paul 1996. Psilocybin Mushrooms of the World: An Identification Guide. Ten Speed Press.
The most comprehensive guide to the world of psilocybin-containing fungi in print. Since it only briefly touches on non-psilocybin-containing species, poisonous or otherwise, it should always be consulted in tandem with a good general field guide whenever attempting to make a field identification.
Edible Mushrooms
Carluccio, Antonio. 2003. The Complete Mushroom Cookbook. Rizzoli.
A beautifully photographed coffee-table guide to cooking with wild and cultivated mushrooms. Every recipe we have tried from its pages was delicious.
Fischer, David W., and Alan E. Bessette. 1992. Edible Wild Mushrooms of North America: A Field-to-Kitchen Guide. University of Texas Press.
A good guide to most of the edible North American mushroom species, as well as most of their poisonous brethren. Nice color photographs and many good recipes.
Hall, Ian R., Steven L. Stephenson, Peter K. Buchanan, Wang Yun, and Anthony L. J. Cole. 2003. Edible and Poisonous Mushrooms of the World. Timber Press.
Tekela, Stan and Karen Shanberg. 1993. Start Mushrooming. Adventure Publications.
The best little guide for the beginning edible mushroom forager. Teaches you how to find six “foolproof ’ edible species: those that have no poisonous look-alikes and simply cannot be mistaken for anything else.
Psilocybe Mushroom History, Culture, and Pharmacology
Gartz, Jochen. 1996. Magic Mushrooms Around the World. LIS Publications.
McKenna, Terence. 1993. True Hallucinations. Harper San Francisco.
Terence McKenna’s account of his adventures in the Columbian Amazon in search of oo-koo-hé, the mysterious tryptamine-containing hallucinogen of the Witoto. Though he never did unveil the drug’s mysteries, he and his band of adventurers did experiment with the Psilocybe cubensis mushrooms they found growing in abundance there. A rip-roaring adventure tale that is highly recommended. Includes a chapter describing how Terence and his brother Dennis worked out the cultivation methods they detailed in their book Psilocybin: Magic Mushroom Grower’s Guide.
Metzner, Ralph, ed. 2004. Teonanácatl: Sacred Mushroom of Visions. Four Trees Press.
Ott, Jonathan. 1996. Pharmacotheon: Entheogenic Drugs,Their Plant Sources and History. 2nd edition. Natural Products Co.
Ott, Jonathan, and Jeremy Bigwood, eds. 1978. Teonanácatl: Hallucinogenic Mushrooms of North America. Madrona Publishers.
Pendell, Dale. 2006. Pharmako/Gnosis: Plant Teachers and the Poison Path. Mercury House.
At long last, the much-anticipated third volume of Dale Pendell’s Pharmako trilogy is in print. This series of books is the most poetic, whimsical, and personal guide to psychoactive plants and chemicals there is.They are unlike any other books on the subject, impossible to describe to the uninitiated, and highly recommended. This final volume covers the visionary plants, and includes a chapter on the Psilocybes.
Perrine, Daniel M. 1996. The Chemistry of Mind Altering Drugs. American Chemical Society.
Rätsch, Christian. 2005. The Encyclopedia of Psychoactive Plants: Ethnopharmacology and its Applications. Park Street Press.
Stamets, Paul. 1982. Psilocybe Mushrooms and Their Allies. Homestead Book Company.
Wasson, R. Gordon. 1957. Seeking the Magic Mushroom. Life Magazine, May 13, 1957 42:19.
Wasson, R. Gordon. 1980. The Wondrous Mushroom: Mycolatry in Mesoamerica. McGraw-Hill.
Cultivation Manuals
Chang, Shu-Ting, and W. Hayes. 1978. The Biology and Cultivation of Edible Mushrooms. Academic Press.
Chang, Shu-Ting, and Philip G. Miles. 1989. Edible Mushrooms and Their Cultivation. CRC Press.
Oei, Peter. 1996. Mushroom Cultivation:With Special Emphasis on Appropriate Techniques for Developing Countries. Backhuys Publishers.
Oss, O.T., and O.N. Oeric. 1992. Psilocybin: Magic Mushroom Grower’s Guide: A Handbook for Psilocybin Enthusiasts. Quick American Publishing Company.
The book that launched the careers of untold numbers of mushroom cultivators, including our own. Essential reading.
Pollock, Steven H. 1977. Magic Mushroom Cultivation (Psychomycological Studies No. 1). Herbal Medicine Research Foundation.
Stamets, Paul. 2004. Mycelium Running: How Mushrooms Can Help Save the World. Ten Speed Press.
Stamets, Paul. 2000. Growing Gourmet and Medicinal Mushrooms. Ten Speed Press.
Stamets, Paul, and J. S. Chilton. 1983. The Mushroom Cultivator: A Practical Guide to Growing Mushrooms At Home. Agarikon Press.
These three titles, more than a thousand pages all told, are the most comprehensive guides to edible, medicinal, and psychoactive mushroom cultivation available.They are unquestionably the next set of titles you should look to when you are ready to try your hand at other methods or species. The Mushroom Cultivator and Growing Gourmet and Medicinal Mushrooms focus on more complex professional cultivation methods, while the most recent title, Mycelium Running, presents a decidedly more low-tech, “organic” approach to mostly outdoor cultivation.
Steineck, Hellmut. 1984. Mushrooms in the Garden. Mad River Press.
Wayne, R. Rush. 2001. Growing Mushrooms the Easy Way: Home Mushroom Cultivation With Hydrogen Peroxide, Volumes 1 & 2. Self-published. (www.mycomasters.com).
Wayne, R. Rush 2004. Non-Sterile Mushroom Cultivation. Self-published. (www.mycomasters.com).
All three of Wayne’s books are essential reading for a complete understanding of the use of peroxide in mushroom cultivation, as well as a wealth of other useful tricks of the trade.
General Mycology Texts
Ainsworth, G. C., P. M. Kirk, Guy Richard Bisby, P. F. Cannon, J. C. David, and J. A. Stalpers. 2001. Ainsworth and Bisby’s Dictionary of Fungi. CABI Publishing.
Alexopoulos, C. J., Charles W. Mims, and M. Blackwell. 1996. Introductory Mycology. Wiley Text Books.
Benjamin, Denis, R. 1995. Mushrooms: Poisons and Panaceas. WH Freeman.
A comprehensive guide to the chemistry of medicinal, edible, psychoactive, and poisonous fungi.
Deacon, J. W. 1997. Modern Mycology. Blackwell Science.
Hobbes, Christopher. 2003. Medicinal Mushrooms:An Exploration of Tradition, Healing, & Culture. Botanica Press.
Hudler, George. 2000. Magical Mushrooms, Mischievous Molds. Princeton University Press.
Kendrick, Bryce. 2001. The Fifth Kingdom. Focus Publishing / R. Pullins & Co.
An excellent, well-written mycology textbook that manages to be both accessible and technically comprehensive. Also available as an enhanced CD-ROM with color photographs and videos.
Money, Nicholas P. 2004. Mr. Bloomfield’s Orchard: the Mysterious World of Mushrooms, Molds, and Mycologists. Oxford University Press.
One of our favorite mycology books by one of our favorite mycology writers. Laugh-out-loud funny at times and always fascinating. Money comes as close as humanly possible to making mycology and those who practice it seem sexy.
Money, Nicholas P. 2004. Carpet Monsters and Killer Spores. Oxford University Press.
More of the same, this time on the subject of molds and “sick building” syndrome.
Moore, David. 2000. Slayers, Saviors, Servants and Sex: An Exposé of Kingdom Fungi. Springer Verlag.
Schaecter, Elio. 1997. In the Company of Mushrooms: A Biologist’s Tale. Harvard University Press.
Magazines and Journals
Mushroom: The Journal of Wild Mushrooming
1511 E. 54th St.
Chicago, IL 60615
www.mushroomthejournal.comA great resource for the amateur mycologist. Contains articles on mushroom collecting, cultivation, books, stamps, recipes, and even a mushroom crossword puzzle.
The Entheogen Review
POB 19820
Sacramento, CA 95819
www.entheogenreview.comThe best stealth journal of psychoactive plants and drugs, period. Contains the occasional (and always informative) article on Psilocybe mushroom cultivation.
Web Sites
Web sites come and go, but we have compiled a list of several that have endured over time, and are likely to continue. Additional resources can be found on a page we maintain on our publisher’s Web site, www.quicktrading.com.
General information:
An excellent source of edible and medicinal mushroom cultivation articles and information.
Rush Wayne’s Web site. In addition to ordering information for all of his books, he posts frequent updates and feedback about the peroxide method.
One of the better mushroom cultivation online communities. Though mainly focused on Psilocybe species, there is a fair bit of discussion about edible and medicinal mushroom cultivation species as well. An excellent place to find reliable and trustworthy sources for spores & supplies.
Unquestionably the most comprehensive and best-researched online resource on psychoactive drugs, plants, and fungi, and their effects. Go here first, and please consider making a donation to support their efforts and keep them up and running.
Spores:
These two sites, one serving North America, the other Europe, are good sources of spore prints from many species of Psilocybe mushrooms, available for the price of postage alone. To keep this valuable service going, consider donating any extra prints you generate to them.
All of the above spore suppliers have been around for some time and have good reputations for customer service and privacy protection.
Mushroom cultivation suppliers:
All of these companies carry any of the specialty cultivation tools and supplies you might not find elsewhere. They cater to a general audience, so please don’t inquire about Psilocybe species.
Appendix D
GLOSSARY
A
agar A polysaccharide (a sugar-like molecule) found in the cell walls of certain algae. When dissolved in boiling water and then cooled, it partially solidifies, like gelatin. Used to create semi-solid media for cul turing fungi and other microorganisms.
agonist A substance that initiates a physiological response when bound to a receptor.
alkaloid Any of a class of nitrogen-containing alkaline organic com pounds of natural origin; many of them have pronounced physiological effects on humans. Psilocybin, morphine, strychnine, and caffeine are all alkaloids.
allelochemical A chemical produced by a living organism that has an effect upon other organisms in its environment.
amine A nitrogen containing, basic organic molecule.
annulus The tissue remnants of the partial veil that remain attached to the stipe as a membranous ring.
antagonist A substance that inhibits or interferes with the action of another substance on a receptor.
aseptic technique The exclusion of living microorganisms from media and the work environment though the use of sterilization and air filtration.
autoclave To sterilize via the use of high pressures and temperatures.
autotroph An organism that is able to form nutritional organic substances from simple inorganic substances such as carbon dioxide.
B
baeocystin 4-phosphoryloxy-N-methyltryptamine, an indole alkaloid produced by many species of Basidiomycete fungi.
Basidiomycetes A class of higher fungi distinguished by having their spores borne on a basidium.
basidium The microscopic club-shaped structure upon which are borne the spores of certain fungi.
binomial The unique two-part Latin name given to a species of organism, consisting of the genus followed by the specific epithet (e.g., Psilocybe cubensis).
biological efficiency The inherent ability of a mushroom species to convert the materials of its substrate into mushrooms. 100% B.E. means a 25% conversion of the wet substrate weight into fresh mushrooms, or 10% of the dry substrate into dried.
bioluminescence The biochemical production of light by living organisms.
C
casing A layer of non-nutritive materials with high water-holding capacities, such as peat moss, coir (finely shredded coconut husks), or vermiculite, applied to a substrate to enhance or promote fruitbody production.
chromosome The threadlike arrangement of genes contained in the nucleus of the cells of most organisms.
clone An organism that is genetically identical to the parent organism from which it was derived.
coprophilic Dung-loving.
cortina A thin, web-like veil extending from the edge of the cap to the stipe on certain mushrooms.
culture The cultivation of microorganisms on artificial media.
D
dikaryotic Containing two nuclei, one from each parent.
diploid Containing a single nucleus with two sets of chromosomes, one from each parent.
E
endospore A resistant asexual spore that develops inside certain bacteria.
F
field capacity The amount of moisture in a material after it has been fully hydrated and drained of any excess.
flush A mushroom crop that forms within a defined time period, often in a regularly repeating manner.
fruit To produce mushrooms.
fruitbody A mushroom.
fruiting substrate The final generation of material prior to fruiting, often identical in composition to secondary spawn. (See also primary spawn, secondary spawn.)
G
gamete A haploid cell capable of mating with another compatible gamete to form a mature diploid or dikaryotic cell.
genetic mosaic An organism that is composed of a mixture of cells of two or more different genotypes.
genotype The particular genetic constitution of an individual organism.
genus The principle taxonomic category ranking above species.
germinate To begin to grow after a period of dormancy.
gills The blade-like structures on the underside of a mushroom cap and upon which spores are borne.
H
haploid Containing a nucleus with a single set of unpaired chromosomes.
heterotroph An organism that derives nutrition from the digestion of complex organic substances.
hymenium The fertile layer of cells on the gill which give rise to basidia and spores.
hypha (pl. -phae) Each of the branching filaments that make up the mycelium of a fungus.
I
indole An aromatic organic compound comprised of a benzene ring fused to a pyrrole ring.
inoculate To introduce an organism onto a medium.
inoculum A substance or material used for inoculation.
K
karyogamy The fusion of parent nuclei during sexual reproduction.
L
lignicolous Growing on or in wood or wood-based materials.
lignin A complex organic polymer that is, along with cellulose, a primary component of woody plants.
Linnaean taxonomy The systematic classification of organisms in a hierarchy.
M
mating type The equivalent in lower organisms of the sexes in higher organisms; the mating types typically differ only physiologically and not in physical form.
meiosis A type of cell division which results in two daughter cells, each containing half of the number of chromosomes as the parent, as in the production of gametes.
mitosis A type of cell division which results in two daughter cells, each containing the same number and kind of chromosomes as the parent.
monokaryotic Comprising cells that contain one haploid nucleus.
mycelium (pl. -ia) A network of fungal hyphae. The primary vegetative state of most fungi.
mycology The study of fungi.
mycorrhiza (pl. -izae) A type of fungus that lives in symbiotic association with plants.
N
nucleus The part of a cell that contains its genetic material.
P
partial veil The thin membrane that extends from the cap margin to the stipe of a mushroom and covers its developing gills.
pileus The mushroom cap.
pin A mushroom primordium.
pinhead A mushroom primordium.
plasmogamy The fusion of the cytoplasm of the cells from two parent mycelia, without fusion of their nuclei. (syn., somatogamy)
primary spawn Sterilized wood spawn that is inoculated from grain cultures in relatively small quantities. (See also fruiting substrate, secondary spawn.)
primordium (pl. -dia) A mushroom at its earliest stage of development. (syn., pin, pinhead)
psilocin 4-hydroxy-N,N-dimethyltryptamine, an indole alkaloid produced by many species of Basidiomycete fungi.
psilocybin 4-phosphoryloxy-N,N-dimethyltryptamine, an indole alkaloid produced by many species of Basidiomycete fungi.
R
rhizomorph Root-like bundles of hyphae formed by many fungi.
S
saprobe An organism that lives by consuming dead or decaying organic matter. (syn., saprophyte)
saprophyte An organism that lives by consuming dead or decaying organic matter. (syn., saprobe)
secondary spawn Spawn derived from primary spawn, and any subsequent expansions thereafter. Unlike primary spawn, secondary spawn is not necessarily made up of sterile materials. (See also fruiting substrate, primary spawn.)
sectoring The appearance of two or more mycelial types within a single culture.
selective Favored by certain organisms and not others.
senescence The degradation or death of a culture due to aging.
sexual reproduction The production of a genetically unique organism from gametes derived from two parent organisms.
somatogamy The fusion of the cytoplasm of the cells from two parent mycelia, without fusion of their nuclei. (syn., plasmogamy)
spawn Any material colonized by the mycelium of a fungus used to inoculate larger quantities of substrate.
spawn rate The ratio of spawn to new substrate.
species A group of organisms capable of interbreeding; the principle taxonomic unit below genus.
spore The minute, often single-celled, reproductive unit of many fungi.
spore print The pattern of spores deposited on a surface from a sporulating mushroom cap.
stem butt The lower portion of a mushroom stipe and any attached substrate.
sterigma (pl. -mata) The horn-like protuberances at the end of a basidium upon which the spores are borne.
sterile technique The exclusion of living microorganisms from media and from the work environment though the use of sterilization and air filtration.
stipe The stem of a mushroom.
strain A genetically unique subset of organisms.
substrate Any material upon which a fungal mycelium will grow.
T
threshold dose The minimum amount of drug required to produce a response.
tryptamine An organic compound comprising an indole ring and an amine, joined by a two-carbon chain.
Index
(Note: bold numbers indicate photos.)
A
agar medium
advantages of
alternating recipes of
“anything,”
definition of
making
malt yeast
agar plates
colonized
contamination on
of P. azurescens
preparing
storing
agar techniques
agar wedges
Agaricus bisporus (common button mushroom)
agar-to-agar transfers (subculturing) to maintain a stored strain
agar-to-grain transfers
agonist molecules
alcohol. See isopropyl alcohol
alcohol extract, making
alcohol lamps
alkaloids
and monoamine oxidase inhibitors See also baeocystin, psilocin, psilocybin
allelochemicals
annulus See also partial veil
antagonist molecules
“anything” agar medium
aseptic culture technique See also sterile culture technique; sterilization techniques
autoclaving
autotrophs
B
bacterial contaminants. See contaminants, bacteria
bacterial endospores
baeocystin
balances
basidia, definition of
role in spore discharge
Basidiomycetes
bioluminescent
definition of
binomial, taxonomic
biology of mushrooms
bleach
“Blue Ringer” mushrooms. See Psilocybe stunzii
bluing reaction
brown rice. See grain substrates, brown rice
brown rot fungi
“bulk” methods of cultivation
button mushrooms (Agaricus bisporus)
C
“cake” method in PF Tek
calcium carbonate (lime)
calcium sulfate (gypsum)
caramel-capped Psilocybes See also wood-loving Psilocybe species; individual species
carbon dioxide buildup
cardboard disc spore germination
cardboard method of tissue transfers
casing layer
and fruiting
and scratching
applying
grass in
history of
in outdoor beds
peat moss as
vermiculite as
water crystals in
casing soils
contamination in
pasteurizing
recipes for
cat litter, paper pellet. See paper pellet cat litter
chalk. See calcium carbonate
cinnamon cap mushrooms
classification of mushrooms
clones See also tissue transfers
cloning. See tissue transfers
cobweb mold
cold-shocking
colonization
grain jar
in PF Tek
of agar plates
of outdoor substrates
of secondary spawn
shaking jars to facilitate
stages of
companion planting. See grass in outdoor casing layer
contaminants
agar
bacteria
brown-rot fungi
cobweb mold (Dactylium dendroides)
dust
mold
outdoor
personal hygiene and
Trichoderma viride
contamination
and casing soils
“capping” to prevent
diagnosing
in agar plates
in grain jars
in outdoor substrates
in spawn bags
resistance from hydrogen peroxide
resistance in wood-based substrates
coprophilic species See also substrates, manure as
cultivation, indoor. See agar techniques; grain techniques; PF Tek
cultivation, outdoor. See outdoor cultivation
culture plates. See agar plates.
cultures
making. See agar techniques
making a master
making multiple
paper pellet
virgin
wild-collected
D
Dactylium dendroides (cobweb mold)
dikariotization
dikaryotic mycelium
diploid nucleus
dosages for use
and tolerance level
by species
levels of
dowels, spiral-grooved
drying mushrooms
E
edible mushrooms
effects of use
and tolerance
based on dosage
from P. azurescens
endospores, bacterial
environmental change and fruiting See also humidity; outdoor cultivation, climates; temperature
equipment
obtaining
selecting
shopping key for
expansion See also life cycle of mushrooms, stages of
F
Falconer, William
field capacity
filter discs
filter patch bags. See spawn bags
flow hood
construction of
flushes
and potency
in PF Tek
See also fruiting
fruitbody initiation. See fruiting, initiating
fruiting
initiating
lights during
outdoors
PF Tek
Psilocybe azurescens
Psilocybe cubensis
fruiting containers
cardboard tubes as
substrate depth in
fruiting substrates
funnels
G
gametes, definition of
genus
germination, spore
on agar
on cardboard discs
gills
role in spore discharge
glove box
definition
construction of
gloves, surgical
graduated cylinders
grain jars
in stages of colonization
labeled
loading
shaking
grain spawn
and contamination
preparation
recipes
viability of
grain spawn bags. See spawn bags
grain substrates
brown rice
comparison of
for agar
inoculating
rye berries
wheat
grain techniques
grain-to-grain transfers
grass in outdoor casing layer
grow racks
gypsum. See calcium sulfate.
H
habitats of Psilocybe mushrooms
haploid nucleus
harvesting
cleaning mushrooms after
in PF Tek
mushrooms at ideal stage for
outdoors
using chopsticks in
Heim, Roger
HEPA filters
heterotrophs
history of methods of cultivation
humidifiers
humidity
and overlay
in outdoor cultivation
levels during fruiting
See also misting; spawn bags, moisture problems in; watering in outdoor
substrates
humidity tent
hydrogen peroxide
and agar-to-grain transfers
and cloning
and grain-to-grain transfers
and long-term storage
and outdoor cultivation
and Petri dishes
and spawn bags
chemistry of
determining concentration of
introduction of
solution for misting
hymenium
hyphae
definition of
growth of
See also mycelium; rhizomorphs
Hypholoma sublateritum (brick or cinnamon caps)
I
impulse sealers
incubation
after scratching
in agar methods
in outdoor cultivation
in PF Tek
of grain jars
of larger containers
inoculation loops
inoculation methods
for grain
for outdoor cultivation
for spawn bags
isolation
isopropyl alcohol
J
jars. See grain jars; mason jars
K
Kingdom Fungi, definition of
L
life cycle of mushrooms, stages of
colonization
expansion
fruiting
germination
isolation
pinning
sexual reproduction
See also colonization; fruiting; germination; isolation; sexual reproduction
lights in fruiting phase
lignicolous species. See wood-loving Psilocybe species
lignin
lime. See calcium carbonate
Linnaean taxonomy
Linnaeus, Carolus
M
malt extract
malt yeast agar medium, making
manure as substrate
mason jars
lids for
mating types
McKenna, Dennis See also Oss, O.T.
McKenna, Terence See also Oeric, O.N.
measuring cups and spoons
media. See agar medium; paper pellet storage medium; substrates
media flasks
methods of ingestion See also potency; safety of use
minitorches
misidentification of mushrooms
misting
after scratching
during fruiting
during harvesting
in PF Tek
See also watering in outdoor substrates
molds. See contaminants, mold
monoamine oxidase inhibitors
monokaryotic mycelium
mushrooms
biology of
bioluminescent
chemistry of
classification of
definition of
digestive system of
edible
foraging for
identifying
ingestion methods
life cycle See also life cycle of mushrooms, stages of
misidentification of
parts of
potency of
preparation for use
preserving
sexual reproduction of
See also primordia; spores; individual species
MYA medium. See malt yeast agar (MYA) medium
mycelial mass
mycelium
cutting out
definition of
dikaryotic
in jars
in subculturing
monokaryotic
on cardboard
on wood chips
overlay in
parent
P. azurescens
transferring See also transfers
See also germination, spore; hyphae; primordia
mycorrhizal fungi
O
Oeric, O.N.
Oss, O .T.
outdoor cultivation
advantages of
and fruiting
and harvesting
“capping” in
choosing location for
climates for See also temperatures for outdoor cultivation
colonization in
dormant period for
germination and
preparing for
restoring depleted beds
starting a new bed
transferring spawn to
oven bags
overlay
oyster mushrooms
P
Panellus stipticus
paper pellet cat litter
paper pellet storage medium
paper pellet storage tubes
parafilm
parasitic fungi
partial veil
cortinate
See also annulus
pasteurization
of casing soil
peat moss
perlite
peroxidases
Petri dishes
alternatives to
care of
resterilizing
PF Tek
basic method of
“cakes” in
colonization
drawbacks of
for wood-loving species
fruiting
harvesting in
improvements to
inoculation
introduction of
making spore syringes for
pileus, definition of
pinning
pipettes
Pleurotus ostreatus (oyster mushroom)
Pollock, Dr. Steven H.
potency
and dosage levels
and methods of ingestion
comparison between species
of P. azurescens
of P. cyanescens
preserving mushrooms
pressure cookers
alternatives to
loading
primary spawn
making
primordia
damaged in harvesting
See also fruiting
psilocin
Psilocybe azurescens
Psilocybe bohemica
Psilocybe cubensis
as beginner mushroom
casing soils for
description of
habitat of
humidity levels for
fruiting
primordia
substrates for
Psilocybe cyanescens, (“wavy caps”)
Psilocybe cyanofibrillosa
Psilocybe Fanaticus
Psilocybe Fanaticus Technique. See PF Tek
Psilocybe serbica
Psilocybe stunzii (Blue Ringers)
Psilocybe subaeruginosa
Psilocybe tasmaniana
psilocybin
Psilocybin: Magic Mushroom Grower’s Guide
R
record keeping
rhizomorphs
S
safety of use
and dosage levels
monoamine oxidase inhibitors and
saprophytes
sawdust
fuel pellets
scalpels
scratching
secondary spawn
making
sectoring
selectivity
senescence, strain
septa
sexual reproduction of mushrooms
and spore prints
sharpies
Sinden, James W.
spawn
definition of
grain
primary
secondary
spawn bags
contaminated
inoculating
loading and cooking
moisture problems in
spawn rates
species, definition of See also individual species
spore germination
on agar
on cardboard discs
spore prints
making
obtaining
starting from
spores
definition of
discharge of
obtaining
starting on agar
spore streaking
spore syringes
in outdoor cultivation
making
Stamets, Paul
sterigma
definition of
role in spore discharge
sterile culture technique
sterilization techniques
and flow hoods
and glove boxes
and outdoor cultivation
history of
water bath
stipe, definition of
storage
long-term strain
of mushrooms
of primary spawn
of secondary spawn
of spore prints
of spore syringes
retrieving cultures from
storage tubes
inoculating
malt yeast extract
paper pellet
Stropharia rugosoannulata (wine-cap stropharia)
subculturing. See agar-to-agar transfers
substrates
comparison of
definition of
depth in fruiting containers
fruiting
grain For specific grains, see grain substrates
manure
mixing
vermiculite in
wood
supplies
surgical gloves
syringes See also spore syringes.
T
tea, making mushroom
temperature
and fruiting
and humidity
and overlay
during incubation
for outdoor cultivation
for storage
increase from mycelium
winter dormancy and
threshold dose
tissue transfers (cloning)
cardboard method of
in outdoor cultivation
versus sexual reproduction
tolerance
toxicity of Psilocybe mushrooms
transfers
agar-to-agar
agar-to-grain
from storage
grain-to-grain
grain-to-wood
minimizing
naturalized spawn
See also tissue tranfers
Trichoderma viride
tryptamines
Tyvek
U
usage
dosages for
effects of
preparation for
safety of
V
vermiculite
as casing layer
in substrates
role of
safety with
W
Wasson, R. Gordon
water bath sterilization
water crystals
watering in outdoor substratesSee also misting
water source
wavy caps mushrooms. See Psilocybe cyanescens
Wayne, Rush
wine cap stropharia mushrooms
winter dormancy
wood-based primary spawn. See primary spawn
wood chips
as substrate
choice of
colonized
resistance to contamination in
wood-loving Psilocybe species See also individual species indoor cultivation of
workspace, preparing
Y
yeast extract

1
Wacky, indeed: we have seen photographs of P. cubensis growing from both U.S. paper currency and a copy of the King James Bible.
2
We were greatly assisted in the writing of this chapter by the article “Mushroom Cultivation, From Falconer to Fanaticus and Beyond,” by Yachaj, from the Winter 2001 issue of Entheogen Review (pp. 127-139). This excellent article covers the history of Psilocybe mushroom cultivation in far greater detail than we do here, and is well worth a look.
3
In Asia, the science of mushroom cultivation was considerably more advanced. The shiitake mushroom (Lentinula edodes) had been propagated for more than a thousand years by placing freshly cut logs beside trees bearing mushrooms, a crude but effective “inoculation” method. 3 Wild-collected “spawn transfer” methods of this kind are quite effective if the substrate is itself naturally resistant to contamination. See chapter 13 for details on how it can be used to create new beds of wood loving Psilocybes.
5
It also spawned a whole new industry: since the spores themselves contain no psilocybin, they are not strictly illegal to possess or sell. A number of entrepreneurs, Mr. Fanaticus among them, have made a good living in the intervening years selling prepared spore-water syringes.
6
Wayne even describes several methods that avoid the need for a pressure cooker altogether, but we have found full sterilization of agar and grain media before the addition of peroxide to be much more reliable in practice.
7
In truth, they cannot be avoided. Fungi are everywhere: in the air you breathe, on your shower curtain, in the soil beneath your feet, even on your feet. Don’t worry, though: 99.99999% of them are harmless to you, and most are quite helpful or even essential. If you knew all that they did to keep the planet functioning properly, you’d be grateful for their presence.
8
While all fungi reproduce, not all fungi produce mushrooms.“Mushroom” is the term we apply to the reproductive structures of fungi when they are more or less large enough to see individually with the naked eye.
10
Until recently, the definition of “biological similarity” was a subject of much debate. The advent of DNA sequencing technology, however, has eliminated most of this ambiguity and forced the reclassification of many species that were once thought more closely related than they actually are.
12
Convention dictates that species binomials are always italicized. In addition, the genus is commonly abbreviated to its first letter followed by a period, particularly when the context makes it clear what name is otherwise implied.
13
Strictly speaking, not all Basidiomycetes act or look quite this way, but all of those we are interested in here, all those of the genus Psilocybe, do.
14
The term “catapult” downplays the actual violence of this miraculous event. The momentum generated by the collapsing droplet is sufficient to give the flying spore an acceleration of 25,000 times the force of gravity. For comparison, the Space Shuttle maxes out somewhere around 2 Gs.
15
Along with a whole host of other spores from other fungi who had the same bright idea and are just as pleased to be there too. But theirs is a story for another day.
16
Such fungi cannot live in the absence of their host. Many delicious edible fungi (truffles and chanterelles, for example) grow only in relationship with specific trees. Mycorrhizal fungi have so far resisted all attempts at cultivation, and can only be collected from the wild, which is why they demand such high prices.
17
As far as we know nobody has yet done a study to determine exactly how many mating types there are for P. cubensis, but the numbers are at least in the hundreds. There’s a perfect research project for you to undertake once you finish this book and decide to pursue a PhD in mycology.
20
Think of these methods as a “sterile culture arsenal.” You don’t need to use each and every one of these methods to succeed, but the more you adopt, the greater your chances will be.
21
This is particularly common when cloning P. cubensis. Some species of mushrooms can in fact contain more than one strain within a single fruit, and are considered “genetic mosaics”. Cloning a mosaic could result in a number of strains of varying characters from a single parent. To our knowledge, no one has yet demonstrated that P. cubensis displays such genetic mosaicism, but our experience suggests it is likely, and worth further investiga-
22
Once again, we have Rush Wayne to thank for this trick, as described in Growing Mushrooms the Easy Way, Volume II.
25
Here’s a single bit of anecdotal evidence for this idea: we once dumped a contaminated jar of P. cubensis spawn into our worm composting bin, and all of the worms were dead within a few weeks. Coincidence? You decide. We got a new batch of worms, and, for their sake, did not repeat the experiment.
26
The number of connections between neurons in the human brain is greater than the number of atoms in the known universe.
27
See the resources section of the appendix for recommended sources. The online drug information site Erowid (www.erowid.org) has compiled an extensive collection of “trip reports” and is an excellent place to begin. For a more select collection of first-hand accounts, we highly recommend the book Teonanácatl: Sacred Mushroom of Visions, edited by Ralph Metzner.
28
Beyond this dose level, we strongly suggest avoiding situations that might bring you in contact with unwitting strangers, for their sake as much as your own.
29
Grocery and health food stores with a good selection of fresh mushrooms are excellent places to forage for new species and strains to cultivate.
30
Optional, added after sterilization & cooling.
31
A 10% bleach/water solution is a mixture of 1 part regular-strength bleach with 9 parts water.
Psilocybin Mushroom Handbook
Copyright © 2006 Lux Natura
eISBN : 978-0-932-55133-7
Library of Congress information available.
S.
The material offered in this book is presented as information that should be available to the public. The Publisher does not advocate breaking the law. However, we urge readers to support the secure passage of fair and sane drug legislation.
All rights reserved. No part of this book may be used or reproduced in any manner whatsoever without written permission of the Publisher, except in the case of brief quotations embodied in critical articles or reviews.